Cadherin interaction probed by atomic force microscopy
- PMID: 10759550
- PMCID: PMC18132
- DOI: 10.1073/pnas.070052697
Cadherin interaction probed by atomic force microscopy
Abstract
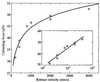
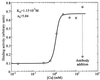
Single molecule atomic force microscopy was used to characterize structure, binding strength (unbinding force), and binding kinetics of a classical cadherin, vascular endothelial (VE)-cadherin, secreted by transfected Chinese hamster ovary cells as cis-dimerized full-length external domain fused to Fc-portion of human IgG. In physiological buffer, the external domain of VE-cadherin dimers is a approximately 20-nm-long rod-shaped molecule that collapses and dissociates into monomers (V-shaped structures) in the absence of Ca(2+). Trans-interaction of dimers is a low-affinity reaction (K(D) = 10(-3)-10(-5) M, k(off) = 1.8 s(-1), k(on) = 10(3)-10(5) M(-1) x s(-1)) with relatively low unbinding force (35-55 pN at retrace velocities of 200-4,000 nm x s(-1)). Higher order unbinding forces, that increase with interaction time, indicate association of cadherins into complexes with cumulative binding strength. These observations favor a model by which the inherently weak unit binding strength and affinity of cadherin trans-interaction requires clustering and cytoskeletal immobilization for amplification. Binding is regulated by low-affinity Ca(2+) binding sites (K(D) = 1.15 mM) with high cooperativity (Hill coefficient of 5.04). Local changes of free extracellular Ca(2+) in the narrow intercellular space may be of physiological importance to facilitate rapid remodeling of intercellular adhesion and communication.
Figures

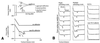

References
-
- Gumbiner G M. Cell. 1996;84:345–357. - PubMed
-
- Takeichi M. Curr Opin Cell Biol. 1995;7:619–627. - PubMed
-
- Lampugnani M G, Dejana E. Curr Opin Cell Biol. 1997;9:674–682. - PubMed
-
- Chothia C, Jones E Y. Annu Rev Biochem. 1997;66:823–862. - PubMed
-
- Shapiro L, Fannon A M, Kwong P D, Thomposn A, Lehmann M S, Grubel G, Legrand J F, Als-Nielsen J, Colman D R, Hendrickson W A. Nature (London) 1995;374:327–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

