Cytotoxic T cell immunity against telomerase reverse transcriptase in humans
- PMID: 10759561
- PMCID: PMC18312
- DOI: 10.1073/pnas.070560797
Cytotoxic T cell immunity against telomerase reverse transcriptase in humans
Abstract
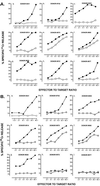
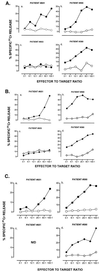
Telomerase is a ribonucleoprotein enzyme which has been linked to malignant transformation in human cells. Telomerase activity is increased in the vast majority of human tumors, making its gene product the first molecule common to all human tumors. The generation of endogenously processed telomerase peptides bound to Class I MHC molecules could therefore target cytotoxic T lymphocytes (CTL) to tumors of different origins. This could advance vaccine therapy against cancer provided that precursor CTL recognizing telomerase peptides in normal adults and cancer patients can be expanded through immunization. We demonstrate here that the majority of normal individuals and patients with prostate cancer immunized in vitro against two HLA-A2.1 restricted peptides from telomerase reverse transcriptase (hTRT) develop hTRT-specific CTL. This suggests the existence of precursor CTL for hTRT in the repertoire of normal individuals and in cancer patients. Most importantly, the CTL of cancer patients specifically lysed a variety of HLA-A2(+) cancer cell lines, demonstrating immunological recognition of endogenously processed hTRT peptides. Moreover, in vivo immunization of HLA-A2.1 transgenic mice generated a specific CTL response against both hTRT peptides. Based on the induction of CTL responses in vitro and in vivo, and the susceptibility to lysis of tumor cells of various origins by hTRT CTL, we suggest that hTRT could serve as a universal cancer vaccine for humans.
Figures

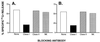
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

