Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters
- PMID: 10759687
- PMCID: PMC2014928
- DOI: 10.1046/j.1365-2125.2000.00164.x
Dose loading with delayed-release mesalazine: a study of tissue drug concentrations and standard pharmacokinetic parameters
Abstract
Aims: Tissue concentrations of 5-aminosalicylic acid (5ASA) and its metabolites may influence the clinical course of inflammatory bowel disease. Since the factors that determine tissue drug concentrations are unknown we have studied the relationships between the oral dose of delayed-release mesalazine, rectal tissue drug concentrations and standard pharmacokinetic parameters.
Methods: Twelve healthy volunteers were studied following 7 days treatment with 1.2, 2.4 and 4.8 g of delayed-release mesalazine daily. 5-aminosalicylic acid and N-acetyl 5-aminosalicylic acid concentrations were measured in serum, urine, stool and rectal tissue biopsies.
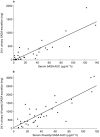
Results: Serum concentrations and 24 h urinary excretion of 5ASA and N-acetyl 5ASA increased as the oral dose of mesalazine was increased from 1.2 g through 2.4 g to 4.8 g daily (serum area under curve (AUC):5ASA = 3. 9, 15.4 and 46.8 microg ml-1 h, P < 0.0001; N-acetyl 5ASA = 17.2, 30. 9 and 57.8 microg ml-1 h, P < 0.0001: urinary excretion: 5ASA = 1.8, 85.5 and 445 mg, P < 0.0001; N-acetyl 5ASA = 250, 524 and 1468 mg, P < 0.0001, respectively). Faecal 5ASA excretion increased as the oral dose increased from 1.2 g to 2.4 g but did not increase further with 4.8 g daily dosing whereas faecal N-acetyl 5ASA excretion was similar at all three doses. Rectal tissue concentrations of 5ASA increased markedly, and N-acetyl 5ASA increased modestly, as the dose of oral mesalazine increased from 1.2 g to 2.4 g daily but neither increased further with 4.8 g daily dosing.
Conclusions: The relationship between the ingested dose of delayed-release mesalazine and rectal tissue drug concentrations is complex. Factors other than dose are likely to be important determinants of rectal tissue drug concentrations.
Figures
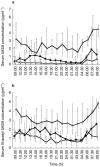

 ) (box and whisker plots) *P < 0.05 and (b) 24 h faecal excretion of 5ASA and N-acetyl 5ASA (box and whisker plots)*P < 0.05.
) (box and whisker plots) *P < 0.05 and (b) 24 h faecal excretion of 5ASA and N-acetyl 5ASA (box and whisker plots)*P < 0.05.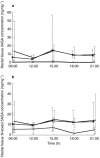
References
-
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977;ii:892–895. - PubMed
-
- Willoughby CP, Cowan RE, Gould SR, Machell RJ, Stewart JB. Double blind comparison of olsalazine and sulphasalazine in active ulcerative colitis. Scand J Gastroenterol. 1988;(Suppl 148):40–44. - PubMed
-
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulphasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterol. 1988;94:1383–1389. - PubMed
-
- Fockens P, Mulder CJ, Tytgat JN, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Eur J Gastroenterol Hepatol. 1995;7:1025–1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

