Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages
- PMID: 10759775
- PMCID: PMC1905630
- DOI: 10.1046/j.1365-2249.2000.01204.x
Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages
Abstract
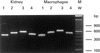
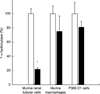
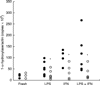
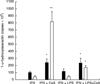
Receptors for 1,25(OH)2vitaminD3 are found in most immune cells and important immunological effects have been described in vitro, reflected by its capacity to prevent autoimmunity and to prolong graft survival. The aim of this study was to examine the presence and nature of the enzyme responsible for final activation of the molecule, 1-alpha-hydroxylase, in murine macrophages and to analyse its regulation and possible role in the immune system. Peritoneal macrophages from C57Bl/6 mice were incubated with lipopolysaccharide (LPS; 100 microg/ml), interferon-gamma (IFN-gamma; 500 U/ml) or a combination of both. By quantitative reverse transcriptase-polymerase chain reaction, using primers based on the murine renal cDNA sequence, low levels of 1-alpha-hydroxylase mRNA were detected in freshly isolated cells (18 +/- 7 x 10-6 copies/beta-actin copies). Analysis of the cDNA sequence of the gene revealed identical coding sequences for the macrophage and renal enzymes. mRNA levels rose three-fold with LPS (NS), but a six-fold increase was seen after IFN-gamma stimulation (P < 0.05). Combining LPS and IFN-gamma did not result in a major additional increase, but addition of cyclosporin A further increased levels 2.5-fold both in IFN-gamma- and combination-stimulated cells (P < 0.05). Time course analysis revealed that up-regulation of 1-alpha-hydroxylase was a late phenomenon, preceded by the up-regulation of activating macrophage products such as IL-1 and tumour necrosis factor-alpha. Finally, a defect in 1-alpha-hydroxylase up-regulation by immune stimuli was found in autoimmune non-obese diabetic mice. In conclusion, we propose that the up-regulation of 1-alpha-hydroxylase in activated macrophages, resulting in the synthesis of 1,25(OH)2D3, might be a negative feedback loop in inflammation. A defect in this system might be an additional element in tipping the balance towards autoimmunity.
Figures

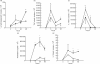
References
-
- Casteels K, Bouillon R, Waer M, Mathieu C. Immunomodulatory effects of 1,25-dihydroxyvitamin D3. Curr Opin Nephrol Hyperten. 1995;4:313–8. - PubMed
-
- Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49:26–31. - PubMed
-
- Hewison M. Vitamin D and the immune system. J Endocrinol. 1992;132:173–5. - PubMed
-
- Provvedini DM, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptor distribution and effects in subpopulations of normal human T lymphocytes. J Clin Endocrinol Metab. 1989;68:774–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

