Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis
- PMID: 10760238
- PMCID: PMC139847
- DOI: 10.1105/tpc.12.4.479
Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis
Abstract
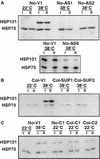
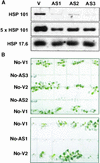
Plants are sessile organisms, and their ability to adapt to stress is crucial for survival in natural environments. Many observations suggest a relationship between stress tolerance and heat shock proteins (HSPs) in plants, but the roles of individual HSPs are poorly characterized. We report that transgenic Arabidopsis plants expressing less than usual amounts of HSP101, a result of either antisense inhibition or cosuppression, grew at normal rates but had a severely diminished capacity to acquire heat tolerance after mild conditioning pretreatments. The naturally high tolerance of germinating seeds, which express HSP101 as a result of developmental regulation, was also profoundly decreased. Conversely, plants constitutively expressing HSP101 tolerated sudden shifts to extreme temperatures better than did vector controls. We conclude that HSP101 plays a pivotal role in heat tolerance in Arabidopsis. Given the high evolutionary conservation of this protein and the fact that altering HSP101 expression had no detrimental effects on normal growth or development, one should be able to manipulate the stress tolerance of other plants by altering the expression of this protein.
Figures






Comment in
-
HSP101: a key component for the acquisition of thermotolerance in plants.Plant Cell. 2000 Apr;12(4):457-60. doi: 10.1105/tpc.12.4.457. Plant Cell. 2000. PMID: 10760235 Free PMC article. Review. No abstract available.
References
-
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. - PubMed
-
- Boston, R.S., Viitanen, P.V., and Vierling, E. (1996). Molecular chaperones and protein folding in plants. Plant Mol. Biol. 32, 191–222. - PubMed
-
- Eleutherio, E.C., Araujo, P.S., and Panek, A.D. (1993). Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology 30, 591–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

