Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model
- PMID: 10760260
- PMCID: PMC18111
- DOI: 10.1073/pnas.97.8.3884
Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model
Abstract
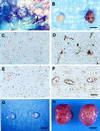
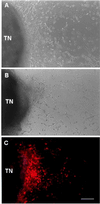
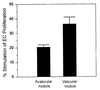
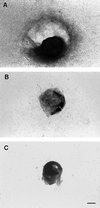
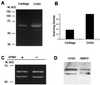
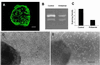
Among the earliest and most important stages during tumorigenesis is the activation of the angiogenic process, an event that is termed the "switch to the angiogenic phenotype." We have developed an in vivo system that can reliably recapitulate the stages in tumor development that represent this transition. Using this model, we have harvested and studied tumor nodules that can be distinguished from each other on the basis of their degree of vascularization. Angiogenic tumor nodules were characterized by the presence of capillary vessels as determined by factor VIII immunohistochemistry, and both angiogenic and proteolytic activities in vitro. In contrast, preangiogenic nodules were devoid of microvessels and showed little angiogenic or proteolytic activity in vitro. Addition of a specific metalloproteinase inhibitor resulted in the abrogation of both angiogenic and proteolytic activities of the angiogenic nodules in vitro. Comparative substrate gel electrophoresis detected the presence of a prominent matrix metalloproteinase (MMP-2) in the angiogenic nodules when compared with the preangiogenic ones. Suppression of MMP-2 activity by antisense oligonucleotides in the vascular nodules resulted in the loss of angiogenic potential both in vitro and in vivo in the chick chorioallantoic membrane assay. Moreover, this suppression of MMP-2 activity in angiogenic nodules inhibited tumor growth in vivo by approximately 70%. These results strongly implicate the activity of MMP-2 as a requirement for the switch to the angiogenic phenotype and validate this model as a reliable and reproducible tool by which to study other cellular and biochemical factors involved in the acquisition of the angiogenic phenotype.
Figures


References
-
- Hanahan D, Folkman J. Cell. 1996;86:353–364. - PubMed
-
- Nagase H, Woessner J F., Jr J Biol Chem. 1999;274:21491–21494. - PubMed
-
- Cockett M I, Murphy G, Birch M L, O'Connell J P, Crabbe T, Millican A T, Hart I R, Docherty A J. Biochem Soc Symp. 1998;63:295–313. - PubMed
-
- Kahari V M, Saarialho-Kere U. Ann Med. 1999;31:34–45. - PubMed
-
- Kleiner D E, Stetler-Stevenson W G. Cancer Chemother Pharmacol. 1999;43,Suppl.:42–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

