The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton
- PMID: 10760273
- PMCID: PMC18134
- DOI: 10.1073/pnas.97.8.4017
The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton
Abstract
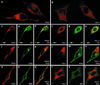
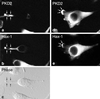
Despite the recent positional cloning of the PKD1 and PKD2 genes, which are mutated in the great majority of patients with autosomal-dominant polycystic kidney disease (PKD), the pathogenic mechanism for cyst formation is still unclear. The finding, that the PKD1 and PKD2 proteins interact with each other through their COOH termini, suggests that both proteins are part of the same protein complex or signal transduction pathway. Using a yeast two-hybrid screen with the PKD2 protein, we isolated the PKD2-interacting protein Hax-1. The specificity of the interaction was demonstrated by the fact that PKD2L, a protein closely related to PKD2, failed to interact with Hax-1. Immunofluorescence experiments showed that in most cells PKD2 and Hax-1 colocalized in the cell body, but in some cells PKD2 and Hax-1 also were sorted into cellular processes and lamellipodia. Furthermore we demonstrated an association between Hax-1 and the F-actin-binding protein cortactin, which suggests a link between PKD2 and the actin cytoskeleton. We speculate that PKD2 is involved in the formation of cell-matrix contacts, which are dysfunctional without a wild-type PKD2 protein, thus leading to cystic enlargement of tubular structures in the kidney, liver, and pancreas.
Figures




References
-
- Gabow P A. N Engl J Med. 1993;329:332–342. - PubMed
-
- The European Polycystic Kidney Disease Consortium. Cell. 1994;77:881–894. - PubMed
-
- Mochizuki T, Wu G, Hayashi T, Xenophontos S L, Veldhuisen B, Saris J J, Reynolds D M, Cai Y, Gabow P A, Pierides A, et al. Science. 1996;272:1339–1342. - PubMed
-
- Hughes J, Ward C J, Peral B, Aspinwall R, Clark K, San Millán J L, Gamble V, Harris P C. Nat Genet. 1995;10:151–159. - PubMed
-
- The International Polycystic Kidney Disease Consortium. Cell. 1995;81:289–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

