Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress
- PMID: 10760305
- PMCID: PMC18252
- DOI: 10.1073/pnas.97.8.4392
Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress
Abstract
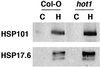
The ability of organisms to acquire thermotolerance to normally lethal high temperatures is an ancient and conserved adaptive response. However, knowledge of cellular factors essential to this response is limited. Acquisition of thermotolerance is likely to be of particular importance to plants that experience daily temperature fluctuations and are unable to escape to more favorable environments. We developed a screen, based on hypocotyl elongation, for mutants of Arabidopsis thaliana that are unable to acquire thermotolerance to high-temperature stress and have defined four separate genetic loci, hot1-4, required for this process. hot1 was found to have a mutation in the heat shock protein 101 (Hsp101) gene, converting a conserved Glu residue in the second ATP-binding domain to a Lys residue, a mutation that is predicted to compromise Hsp101 ATPase activity. In addition to exhibiting a thermotolerance defect as assayed by hypocotyl elongation, 10-day-old hot1 seedlings were also unable to acquire thermotolerance, and hot1 seeds had greatly reduced basal thermotolerance. Complementation of hot1 plants by transformation with wild-type Hsp101 genomic DNA restored hot1 plants to the wild-type phenotype. The hot mutants are the first mutants defective in thermotolerance that have been isolated in a higher eukaryote, and hot1 represents the first mutation in an Hsp in any higher plant. The phenotype of hot1 also provides direct evidence that Hsp101, which is required for thermotolerance in bacteria and yeast, is also essential for thermotolerance in a complex eukaryote.
Figures
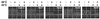



References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

