Natural genetic competence in Bacillus subtilis natto OK2
- PMID: 10762239
- PMCID: PMC111301
- DOI: 10.1128/JB.182.9.2411-2415.2000
Natural genetic competence in Bacillus subtilis natto OK2
Abstract
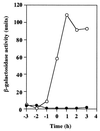
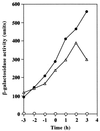
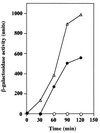
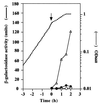
We isolated a Bacillus subtilis natto strain, designated OK2, from a lot of commercial fermented soybean natto and studied its ability to undergo natural competence development using a comG-lacZ fusion at the amyE locus. Although transcription of the late competence genes was not detected in the B. subtilis natto strain OK2 during competence development, these genes were constitutively transcribed in the OK2 strain carrying either the mecA or the clpC mutation derived from B. subtilis 168. In addition, both OK2 mutants exhibited high transformation frequencies, comparable with that observed for B. subtilis 168. Moreover, as expected from these results, overproduction of ComK derived from strain 168 in strain OK2 resulted in a high transformation frequency as well as in induction of the late competence genes. These results clearly indicated that ComK produced in both the mecA and clpC mutants of strain OK2 (ComK(OK2)) could activate the transcription of the whole set of late competence genes and suggested that ComK(OK2) was not activated in strain OK2 during competence development. We therefore sequenced the comS gene of OK2 and compared it with that of 168. The comS(OK2) had a single-base change, resulting in the replacement of Ser (strain 168) by Cys (strain OK2) at position 11.
Figures

References
-
- Birnboim H C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. - PubMed
-
- Dubnau D. Genetic exchange and homologous recombination. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 555–584.
-
- Dubnau D. Binding and transport of transforming DNA by Bacillus subtilis: the role of type-IV pilin-like proteins—a review. Gene. 1997;192:191–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

