Structure, stability and function of RNA pseudoknots involved in stimulating ribosomal frameshifting
- PMID: 10764589
- PMCID: PMC7126452
- DOI: 10.1006/jmbi.2000.3668
Structure, stability and function of RNA pseudoknots involved in stimulating ribosomal frameshifting
Abstract
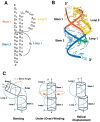
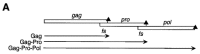
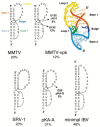
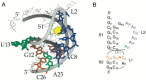
Programmed -1 ribosomal frameshifting has become the subject of increasing interest over the last several years, due in part to the ubiquitous nature of this translational recoding mechanism in pathogenic animal and plant viruses. All cis-acting frameshift signals encoded in mRNAs are minimally composed of two functional elements: a heptanucleotide "slippery sequence" conforming to the general form X XXY YYZ, followed by an RNA structural element, usually an H-type RNA pseudoknot, positioned an optimal number of nucleotides (5 to 9) downstream. The slippery sequence itself promotes a low level ( approximately 1 %) of frameshifting; however, downstream pseudoknots stimulate this process significantly, in some cases up to 30 to 50 %. Although the precise molecular mechanism of stimulation of frameshifting remains poorly understood, significant advances have been made in our knowledge of the three-dimensional structures, thermodynamics of folding, and functional determinants of stimulatory RNA pseudoknots derived from the study of several well-characterized frameshift signals. These studies are summarized here and provide new insights into the structural requirements and mechanism of programmed -1 ribosomal frameshifting.
Copyright 2000 Academic Press.
Figures


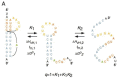
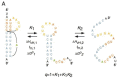

References
-
- Alam S.L, Wills N.M, Ingram J.A, Atkins J.F, Gesteland R.F. Structural studies of the RNA pseudoknot required for readthrough of the gag-termination codon of murine leukemia virus. J. Mol. Biol. 1999;288:837–852. - PubMed
-
- Ban N, Nissen P, Hansen J, Capel M, Moore P.B, Steitz T.A. Placement of protein and RNA structures into a 5 Å-resolution map of the 50 S ribosomal subunit. Nature. 1999;400:841–847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

