Effects of glucagon-like peptide-1(7-36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans
- PMID: 10764704
- PMCID: PMC1727942
- DOI: 10.1136/gut.46.5.622
Effects of glucagon-like peptide-1(7-36)amide on antro-pyloro-duodenal motility in the interdigestive state and with duodenal lipid perfusion in humans
Abstract
Background: Glucagon-like peptide-1(7-36)amide (GLP-1) is a gut hormone released postprandially. Synthetic GLP-1 strongly inhibits gastric emptying in healthy subjects and in patients with diabetes mellitus.
Aims: To investigate the effects of GLP-1 on antro-pyloro-duodenal motility in humans.
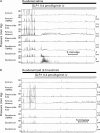
Methods: Eleven healthy male volunteers were studied on two separate days. On the interdigestive study day, a basal period was followed by a 60 minute period of saline infusion and two further 60 minute periods of intravenous infusion of GLP-1 0.4 and 1.2 pmol/kg/min to achieve postprandial and supraphysiological plasma levels, respectively. On the postprandial study day, the same infusions were coadministered with intraduodenal lipid perfusion at 2.5 ml/min (2.5 kcal/min) followed by another 60 minutes of recording after cessation of GLP-1. Antro-pyloro-duodenal motility was measured by perfusion manometry.
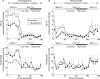
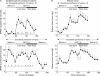
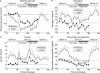
Results: GLP-1 significantly inhibited the number and amplitudes of antral and duodenal contractions in the interdigestive state and after administration of duodenal lipid. It abolished interdigestive antral wave propagation. In the interdigestive state, GLP-1 dose dependently increased pyloric tone and significantly stimulated isolated pyloric pressure waves (IPPW). Pyloric tone increased with duodenal lipid, and this was further enhanced by GLP-1. GLP-1 transiently restored the initial IPPW response to duodenal lipid which had declined with lipid perfusion. Plasma levels of pancreatic polypeptide were dose dependently diminished by GLP-1 with and without duodenal lipid.
Conclusions: GLP-1 inhibited antro-duodenal contractility and stimulated the tonic and phasic motility of the pylorus. These effects probably mediate delayed gastric emptying. Inhibition of efferent vagal activity may be an important mechanism. As postprandial plasma levels of GLP-1 are sufficient to appreciably affect motility, we believe that endogenous GLP-1 is a physiological regulator of motor activity in the antro-pyloro-duodenal region.
Figures


Comment in
-
GLP-1 and the gut.Gut. 2000 May;46(5):591. doi: 10.1136/gut.46.5.591. Gut. 2000. PMID: 10764696 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
