Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice
- PMID: 10766741
- PMCID: PMC316493
Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice
Abstract
Phosphorylation of IkappaB, an inhibitor of NF-kappaB, is an important step in the activation of the transcription factor NF-kappaB. Phosphorylation is mediated by the IkappaB kinase (IKK) complex, known to contain two catalytic subunits: IKKalpha and IKKbeta. A novel, noncatalytic component of this kinase complex called NEMO (NF-kappaB essential modulator)/IKKgamma was identified recently. We have generated NEMO/IKKgamma-deficient mice by gene targeting. Mutant embryos die at E12.5-E13.0 from severe liver damage due to apoptosis. NEMO/IKKgamma-deficient primary murine embryonic fibroblasts (MEFs) lack detectable NF-kappaB DNA-binding activity in response to TNFalpha, IL-1, LPS, and Poly(IC) and do not show stimulus-dependent IkappaB kinase activity, which correlates with a lack of phosphorylation and degradation of IkappaBalpha. Consistent with these data, mutant MEFs show increased sensitivity to TNFalpha-induced apoptosis. Our data provide in vivo evidence that NEMO/IKKgamma is the first essential, noncatalytic component of the IKK complex.
Figures
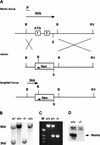

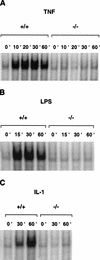
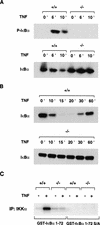
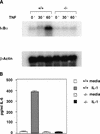


References
-
- Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. - PubMed
-
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. - PubMed
-
- Cohen L, Henzel WJ, Baeuerle PA. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:292–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
