Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells
- PMID: 10766922
- PMCID: PMC2269880
- DOI: 10.1111/j.1469-7793.2000.00415.x
Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells
Abstract
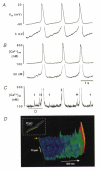
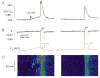
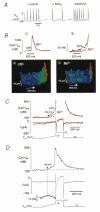
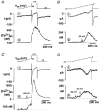
1. The cellular mechanisms governing cardiac atrial pacemaker activity are not clear. In the present study we used perforated patch voltage clamp and confocal fluorescence microscopy to study the contribution of intracellular Ca2+ release to automaticity of pacemaker cells isolated from cat right atrium. 2. In spontaneously beating pacemaker cells, an increase in subsarcolemmal intracellular Ca2+ concentration occurred concomitantly with the last third of diastolic depolarization due to local release of Ca2+ from the sarcoplasmic reticulum (SR), i.e. Ca2+ sparks. Nickel (Ni2+; 25-50 microM), a blocker of low voltage-activated T-type Ca2+ current ((ICa,T), decreased diastolic depolarization, prolonged pacemaker cycle length and suppressed diastolic Ca2+ release. 3. Voltage clamp analysis indicated that the diastolic Ca2+ release was voltage dependent and triggered at about -60 mV. Ni2+ suppressed low voltage-activated Ca2+ release. Moreover, low voltage-activated Ca2+ release was paralleled by a slow inward current presumably due to stimulation of Na+-Ca2+ exchange (INa-Ca). Low voltage-activated Ca2+ release was found in both sino-atrial node and latent atrial pacemaker cells but not in working atrial myocytes. 4. These findings suggest that low voltage-activated ICa,T triggers subsarcolemmal Ca2+ sparks, which in turn stimulate INa-Ca to depolarize the pacemaker potential to threshold. This novel mechanism indicates a pivotal role for ICa,T and subsarcolemmal intracellular Ca2+ release in normal atrial pacemaker activity and may contribute to the development of ectopic atrial arrhythmias.
Figures
Comment in
-
What determines the initiation of the heartbeat?J Physiol. 2000 Apr 15;524 Pt 2(Pt 2):316. doi: 10.1111/j.1469-7793.2000.00316.x. J Physiol. 2000. PMID: 10766913 Free PMC article.
References
-
- Campbell DL, Rasmusson RL, Strauss HC. Ionic current mechanisms generating vertebrate primary cardiac pacemaker activity at the single cell level: an integrative view. Annual Review of Physiology. 1992;54:279–302. - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

