Evaluation of in vivo biocompatibility of different devices for interventional closure of the patent ductus arteriosus in an animal model
- PMID: 10768911
- PMCID: PMC1760843
- DOI: 10.1136/heart.83.5.570
Evaluation of in vivo biocompatibility of different devices for interventional closure of the patent ductus arteriosus in an animal model
Abstract
Objective: To evaluate the in vivo biocompatibility of three different devices following interventional closure of a patent ductus arteriosus (PDA) in an animal model.
Materials and methods: A medical grade stainless steel coil (n = 8), a nickel/titanium coil (n = 10), and a polyvinylalcohol foam plug knitted on a titanium wire frame (n = 11) were used for interventional closure of PDA in a neonatal lamb model. The PDA had been maintained by repetitive angioplasty. Between one and 278 days after implantation the animals were killed and the ductal block removed. In addition to standard histology and scanning electron microscopy, immunohistochemical staining for biocompatibility screening was also undertaken.
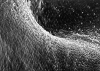
Results: Electron microscopy revealed the growth of a cellular layer in a cobblestone pattern on the implant surfaces with blood contact, which was completed as early as five weeks after implantation of all devices. Immunohistochemical staining of these superficial cells showed an endothelial cell phenotype. After initial thrombus formation causing occlusion of the PDA after implantation there was ingrowth of fibromuscular cells resembling smooth muscle cells. Transformation of thrombotic material was completed within six weeks in the polyvinylalcohol plug and around the nickel/titanium coil, and within six months after implantation of the stainless steel coil. An implant related foreign body reaction was seen in only one of the stainless steel coil specimens and in two of the nickel/titanium coil specimens.
Conclusion: After implantation, organisation of thrombotic material with ingrowth of fibromuscular cells was demonstrated in a material dependent time pattern. The time it took for endothelium to cover the implants was independent of the type of implant. Little or no inflammatory reaction of the surrounding tissue was seen nine months after implantation.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
