Nitric oxide participation in the fungicidal mechanism of gamma interferon-activated murine macrophages against Paracoccidioides brasiliensis conidia
- PMID: 10768942
- PMCID: PMC97457
- DOI: 10.1128/IAI.68.5.2546-2552.2000
Nitric oxide participation in the fungicidal mechanism of gamma interferon-activated murine macrophages against Paracoccidioides brasiliensis conidia
Abstract
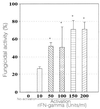
Paracoccidioidomycosis, a systemic mycosis restricted to Latin America and produced by the dimorphic fungus Paracoccidioides brasiliensis, is probably acquired by inhalation of conidia produced by the mycelial form. The macrophage (Mphi) represents the major cell defense against this pathogen; when activated with gamma interferon (IFN-gamma), murine Mphis kill the fungus by an oxygen-independent mechanism. Our goal was to determine the role of nitric oxide in the fungicidal effect of Mphis on P. brasiliensis conidia. The results revealed that IFN-gamma-activated murine Mphis inhibited the conidium-to-yeast transformation process in a dose-dependent manner; maximal inhibition was observed in Mphis activated with 50 U/ml and incubated for 96 h at 37 degrees C. When Mphis were activated with 150 to 200 U of cytokine per ml, the number of CFU was 70% lower than in nonactivated controls, indicating that there was a fungicidal effect. The inhibitory effect was reversed by the addition of anti-IFN-gamma monoclonal antibodies. Activation by IFN-gamma also enhanced Mphi nitric oxide production, as revealed by increasing NO(2) values (8 +/- 3 microM in nonactivated Mphis versus 43 +/- 13 microM in activated Mphis). The neutralization of IFN-gamma also reversed nitric oxide production at basal levels (8 +/- 5 microM). Additionally, we found that there was a significant inverse correlation (r = -0.8975) between NO(2)(-) concentration and transformation of P. brasiliensis conidia. Additionally, treatment with any of the three different nitric oxide inhibitors used (arginase, N(G)-monomethyl-L-arginine, and aminoguanidine), reverted the inhibition of the transformation process with 40 to 70% of intracellular yeast and significantly reduced nitric oxide production. These results show that IFN-gamma-activated murine Mphis kill P. brasiliensis conidia through the L-arginine-nitric oxide pathway.
Figures





References
-
- Bocca A L, Hayashi E E, Pinheiro A G, Furianetto A B, Campanelli A P, Cunha F Q, Figueiredo F. Treatment of Paracoccidioides brasiliensis-infected mice with a nitric oxide inhibitor prevents the failure of cell-mediated immune response. J Immunol. 1998;161:3056–3063. - PubMed
-
- Bocca A L, Silva M F, Silva C L, Cunha F Q, Figueiredo F. Macrophage expression of class II major histocompatibility complex gene products in Paracoccidioides brasiliensis-infected mice. Am J Trop Med Hyg. 1999;61:280–287. - PubMed
-
- Bogdan C, Stenger S, Rollinghof M, Solbach W. Cytokine interactions in experimental cutaneous leishmaniasis. Interleukin-4 synergizes with interferon-γ to activate murine macrophages for killing of Leishmania major amastigotes. Eur J Immunol. 1991;21:327–333. - PubMed
-
- Brummer E. Interaction of Paracoccidioides brasiliensis with host defense cells. In: Franco M, Silva Lacaz C, Restrepo Moreno A, del Negro G, editors. Paracoccidioidomycosis—1994. Boca Raton, Fla: CRC Press; 1994. pp. 213–223.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

