In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection
- PMID: 10768984
- PMCID: PMC97499
- DOI: 10.1128/IAI.68.5.2870-2879.2000
In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection
Abstract
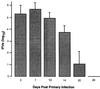
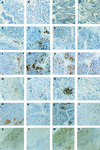
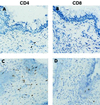
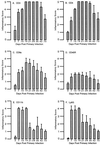
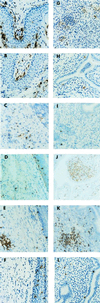
Adaptive immune responses contribute to the resolution of Chlamydia trachomatis genital tract infection and protect against reinfection, but our understanding of the mechanisms of those protective responses is incomplete. In this study, we analyzed by in situ immunohistochemistry the progression of the inflammatory and cytokine responses in the genital tracts of mice vaginally infected with C. trachomatis strain mouse pneumonitis. The cellular inflammatory response was characterized by an initial elevation in myeloid cells in the vagina (day 3) and uterine horns (day 7), followed by a marked rise in the number of T cells, predominantly CD4(+) cells. CD8(+) T cells and CD45R(+) B cells were also detected but were much less numerous. Perivascular clusters of CD4(+) T cells, which resembled clusters of T cells seen in delayed-type hypersensitivity responses, were evident by 2 weeks postinfection. Following the resolution of infection, few CD8(+) T cells and CD45R(+) B cells remained, whereas numerous CD4(+) T cells and perivascular clusters of CD4(+) T cells persisted in genital tract tissues. Interleukin-12 (IL-12)- and tumor necrosis factor alpha (TNF-alpha)-producing cells were observed in vaginal tissue by day 3 of infection and in uterine tissues by day 7. Cells producing IL-4 or IL-10 were absent from vaginal tissues at day 3 of infection but were present in uterine tissues by day 7 and were consistently more numerous than IL-12- and TNF-alpha-producing cells. Thus, the evolution of the local inflammatory response was characterized by the accumulation of CD4(+) T cells into perivascular clusters and the presence of cells secreting both Th1- and Th2-type cytokines. The persistence of CD4(+)-T-cell clusters long after infection had resolved (day 70) may provide for a readily mobilizable T-cell response by which previously infected animals can quickly respond to and control a secondary infectious challenge.
Figures



Comment in
-
Murine models of Chlamydia trachomatis genital tract infection: use of mouse pneumonitis strain versus human strains.Infect Immun. 2000 Dec;68(12):7209-11. doi: 10.1128/IAI.68.12.7209-7211.2000. Infect Immun. 2000. PMID: 11203323 Free PMC article. No abstract available.
References
-
- Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

