Cytoplasmic localization and the choice of ligand determine aggregate formation by androgen receptor with amplified polyglutamine stretch
- PMID: 10769019
- PMCID: PMC2175165
- DOI: 10.1083/jcb.149.2.255
Cytoplasmic localization and the choice of ligand determine aggregate formation by androgen receptor with amplified polyglutamine stretch
Abstract
Polyglutamine tract expansion in androgen receptor is a recognized cause of spinal and bulbar muscular atrophy (SBMA), an X-linked motor neuronopathy. Similar mutations have been identified in proteins associated with other neurodegenerative diseases. Recent studies have shown that amplified polyglutamine repeat stretches form cellular aggregates that may be markers for these neurodegenerative diseases. Here we describe conditions that lead to aggregate formation by androgen receptor with polyglutamine stretch amplification. In transfection experiments, the mutant, compared with the wild-type receptor, was delayed in its cytoplasmic-nuclear translocation and formed large cytoplasmic aggregates in the presence of androgen. The cytoplasmic environment appears crucial for this aggregation, since retention of both the wild-type and mutant receptors in this cellular compartment by the deletion of their nuclear localization signals resulted in massive aggregation. Conversely, rapid nuclear transport of both receptors brought about by deletion of their ligand binding domains did not result in aggregate formation. However, androgen antagonists that altered the conformation of the ligand binding domain and promoted varying rates of cytoplasmic-nuclear translocation all inhibited aggregate formation. This demonstrates that in addition to the cytoplasmic localization, a distinct contribution of the ligand binding domain of the receptor is necessary for the aggregation. The finding that antiandrogens inhibit aggregate formation may provide the basis for in vivo determination of the role of these structures in SBMA.
Figures



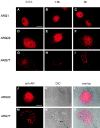
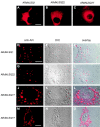

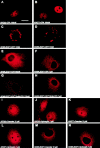
References
-
- Bingham P.M., Scott M.O., Wang S., McPhaul M.J., Wilson E.M., Garbern J.Y., Merry D.E., Fischbeck K.H. Stability of an expanded trinucleotide repeat in the androgen receptor gene in transgenic mice. Nat. Genet. 1995;9:191–196. - PubMed
-
- Butler R., Leigh P.N., McPhaul M.J., Gallo J.-M. Truncated forms of the androgen receptor are associated with polyglutamine expansion in X-linked spinal and bulbar muscular atrophy. Hum. Mol. Genet. 1998;7:121–127. - PubMed
-
- Culig Z., Hobisch A., Cronauer M.V., Cato A.C.B., Hitmair A., Radmayr C., Eberle J., Bartsch G., Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol. Endocrinol. 1993;7:1541–1550. - PubMed
-
- Danek A., Witt T.N., Mann K., Schweikert H.U., Romalo G., La Spada A.R., Fischbeck K.H. Decrease in androgen binding and effect of androgen treatment in a case of X-linked bulbospinal neuronopathy. Clin. Investig. 1994;72:892–897. - PubMed

