The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells
- PMID: 10769025
- PMCID: PMC2175166
- DOI: 10.1083/jcb.149.2.317
The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells
Abstract
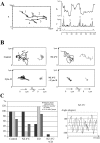
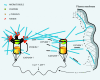
We have generated several stable cell lines expressing GFP-labeled centrin. This fusion protein becomes concentrated in the lumen of both centrioles, making them clearly visible in the living cell. Time-lapse fluorescence microscopy reveals that the centriole pair inherited after mitosis splits during or just after telophase. At this time the mother centriole remains near the cell center while the daughter migrates extensively throughout the cytoplasm. This differential behavior is not related to the presence of a nucleus because it is also observed in enucleated cells. The characteristic motions of the daughter centriole persist in the absence of microtubules (Mts). or actin, but are arrested when both Mts and actin filaments are disrupted. As the centrioles replicate at the G1/S transition the movements exhibited by the original daughter become progressively attenuated, and by the onset of mitosis its behavior is indistinguishable from that of the mother centriole. While both centrioles possess associated gamma-tubulin, and nucleate similar number of Mts in Mt repolymerization experiments. during G1 and S only the mother centriole is located at the focus of the Mt array. A model, based on differences in Mt anchoring and release by the mother and daughter centrioles, is proposed to explain these results.
Figures








References
-
- Andersen S.S.L. Molecular characteristics of the centrosome. Int. Rev. Cytol. 1999;187:51–109. - PubMed
-
- Baron A.T., Greenwood T.M., Bazinet C.W., Salisbury J.L. Centrin is a component of the pericentriolar lattice. Biol. Cell. 1992;76:383–388. - PubMed
-
- Baron A.T., Suman V.J., Nemeth E., Salisbury J.L. The pericentriolar lattice of PtK2 cells exhibits temperature and calcium-modulated behavior. J. Cell Sci. 1994;107:2993–3003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

