Suppression of Pyk2 kinase and cellular activities by FIP200
- PMID: 10769033
- PMCID: PMC2175150
- DOI: 10.1083/jcb.149.2.423
Suppression of Pyk2 kinase and cellular activities by FIP200
Abstract
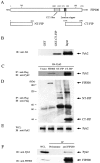
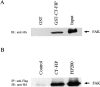
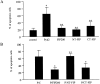
Proline-rich tyrosine kinase 2 (Pyk2) is a cytoplasmic tyrosine kinase implicated to play a role in several intracellular signaling pathways. We report the identification of a novel Pyk2-interacting protein designated FIP200 (FAK family kinase-interacting protein of 200 kD) by using a yeast two-hybrid screen. In vitro binding assays and coimmunoprecipitation confirmed association of FIP200 with Pyk2, and similar assays also showed FIP200 binding to FAK. However, immunofluorescent staining indicated that FIP200 was predominantly localized in the cytoplasm. FIP200 bound to the kinase domain of Pyk2 and inhibited its kinase activity in in vitro kinase assays. FIP200 also inhibited the kinase activity of the Pyk2 isolated from SYF cells (deficient in Src, Yes, and Fyn expression) and the Pyk2 mutant lacking binding site for Src, suggesting that it regulated Pyk2 kinase directly rather than affecting the associated Src family kinases. Consistent with its inhibitory effect in vitro, FIP200 inhibited activation of Pyk2 and Pyk2-induced apoptosis in intact cells, which correlated with its binding to Pyk2. Finally, activation of Pyk2 by several biological stimuli correlated with the dissociation of endogenous FIP200-Pyk2 complex, which provided further support for inhibition of Pyk2 by FIP200 in intact cells. Together, these results suggest that FIP200 functions as an inhibitor of Pyk2 via binding to its kinase domain.
Figures


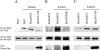

References
-
- Astier A., Avraham H., Manie S.N., Groopman J., Canty T., Avraham S., Freedman A.S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 1997;272:228–232. - PubMed
-
- Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L.M., White R.A., Groopman J.E. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 1995;270:27742–27751. - PubMed
-
- Brinson A.E., Harding T., Diliberto P.A., He Y., Li X., Hunter D., Herman B., Earp H.S., Graves L.M. Regulation of a calcium-dependent tyrosine kinase in vascular smooth muscle cells by angiotensin II and platelet-derived growth factor. Dependence on calcium and the actin cytoskeleton. J. Biol. Chem. 1998;273:1711–1718. - PubMed
-
- Cary L.A., Chang J.F., Guan J.L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996;109:1787–1794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

