Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability
- PMID: 10770229
- PMCID: PMC6871916
- DOI: 10.1002/(sici)1097-0193(200004)9:4<192::aid-hbm2>3.0.co;2-y
Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability
Abstract
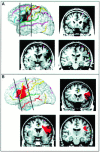
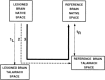
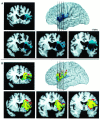
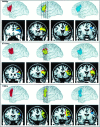
The study of subjects with acquired brain damage has been an invaluable tool for exploring human brain function, and the description of lesion locations within and across subjects is an important component of this method. Such descriptions usually involve the separation of lesioned from nonlesioned tissue (lesion segmentation) and the description of the lesion location in terms of a standard anatomical reference space (lesion warping). The objectives of this study were to determine the sources and magnitude of variability involved in lesion segmentation and warping using the MAP-3 approach. Each of two observers segmented the lesion volume in ten brain-damaged subjects twice, so as to permit pairwise comparisons of both intra- and interobserver agreement. The segmented volumes were then warped to a reference brain using both a manual (MAP-3) and an automated (AIR-3) technique. Observer agreement between segmented and warped volumes was analyzed using four measures: volume size, distance between the volume surfaces, percentage of nonoverlapping voxels, and percentage of highly discrepant voxels. The techniques for segmentation and warping produced high agreement within and between observers. For example, in most instances, the warped volume surfaces created by different observers were separated by less than 3 mm. The performance of the automated warping technique compared favorably to the manual technique in most subjects, although important exceptions were found. Overall, these results establish benchmark parameters for expert and automated lesion transfer, and indicate that a high degree of confidence can be placed in the detailed anatomical interpretation of focal brain damage based upon the MAP-3 technique.
Figures



References
-
- Bedell BJ, Narayana PA, Wolinsky JS. 1997. A dual approach for minimizing false lesion classifications on magnetic resonance images. Magn Reson Med 37: 94–102. - PubMed
-
- Bendszus M, Urbach H, Meyer B, Schultheiss R, Solymosi L. 1997. Improved CT diagnosis of acute middle cerebral artery territory infarcts with density‐difference analysis. Neuroradiology 39: 127–131. - PubMed
-
- Christensen GE, Joshi SC, Miller MI. 1997. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging 16: 864–877. - PubMed
-
- Collins DL, Holmes CJ, Peters TM, Evans AE. 1995. Automatic 3‐D model‐based neuroanatomical segmentation. Hum Brain Mapp 3: 190–208.
-
- Damasio H, Damasio AR. 1980. The anatomical basis of conduction aphasia. Brain 103: 337–350. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

