Efficacy of SCH27899 in an animal model of Legionnaires' disease using immunocompromised A/J mice
- PMID: 10770771
- PMCID: PMC89864
- DOI: 10.1128/AAC.44.5.1333-1336.2000
Efficacy of SCH27899 in an animal model of Legionnaires' disease using immunocompromised A/J mice
Abstract
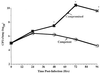
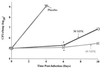
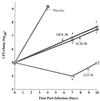
The efficacy of SCH27899, a new everninomicin antibiotic, against replicative Legionella pneumophila lung infections in an immunocompromised host was evaluated using a murine model of Legionnaires' disease. A/J mice were immunocompromised with cortisone acetate and inoculated intratracheally with L. pneumophila serogroup 1 (10(5) CFU per mouse). At 24 h postinoculation, mice were administered either SCH27899 (6 to 60 mg/kg [MPK] intravenously) or a placebo once daily for 5 days, and mortality and intrapulmonary growth of L. pneumophila were assessed. In the absence of SCH27899, there was 100% mortality in L. pneumophila-infected mice, with exponential intrapulmonary growth of the bacteria. In contrast, administration of SCH27899 at a dose of > or =30 MPK resulted in > or =90% survival of infected mice, which was associated with inhibition of intrapulmonary growth of L. pneumophila. In subsequent studies, the efficacy of SCH27899 was compared to ofloxacin (OFX) and azithromycin (AZI). Administration of SCH27899, OFX, or AZI at a dose of > or =30 MPK once daily for 5 days resulted in > or =85% survival of infected mice and inhibition of intrapulmonary growth of the bacteria. However, L. pneumophila CFU were recovered in lung homogenates following cessation of therapy with all three antibiotics. These studies demonstrate that SCH27899 effectively prevents fatal replicative L. pneumophila lung infection in immunocompromised A/J mice by inhibition of intrapulmonary growth of the bacteria. However, in this murine model of pulmonary legionellosis, SCH27899, like OFX and AZI, was bacteriostatic.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

