Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit
- PMID: 10770777
- PMCID: PMC89870
- DOI: 10.1128/AAC.44.5.1356-1358.2000
Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit
Abstract
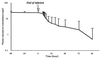
Cerebrospinal fluid (CSF) penetration and the pharmacokinetics of vancomycin were studied after continuous infusion (50 to 60 mg/kg of body weight/day after a loading dose of 15 mg/kg) in 13 mechanically ventilated patients hospitalized in an intensive care unit. Seven patients were treated for a sensitive bacterial meningitis and the other six patients, who had a severe concomitant neurologic disease with intracranial hypertension, were treated for various infections. Vancomycin CSF penetration was significantly higher (P < 0.05) in the meningitis group (serum/CSF ratio, 48%) than in the other group (serum/CSF ratio, 18%). Vancomycin pharmacokinetic parameters did not differ from those obtained with conventional dosing. No adverse effect was observed, in particular with regard to renal function.
Figures
References
-
- Barois A, Estournet B, Moranne J B, Piliot J, Chabenat C, Bataille J. Infections ventriculaires à staphylocoque. Traitement par la vancomycine en perfusion continue. Presse Med. 1986;15:1805–1808. - PubMed
-
- Brinquin L, Rousseau J M, Boulesteix G, Diraison Y, Bonsignour J P. Vancomycine en perfusion continue dans les méningites à staphylocoques post-neurochirurgicales de l'adulte. Presse Med. 1993;22:1815–1817. - PubMed
-
- Desplaces N, Mamoudy P, Ducroquet F, Larrouturou P, Kitsis M D. Vancomycine en perfusion continue dans le traitement des infections ostéo-articulaires chroniques à staphylocoques multirésistants. Med Mal Infect. 1997;27:969–974.
-
- Geraci J E, Heilman F R, Nichols D R, Wellman W E, Ross G T. Some laboratory and clinical experiences with a new antibiotic, vancomycin. Proc Staff Meet Mayo Clin. 1956;31:564–582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical