Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine
- PMID: 10770804
- PMCID: PMC2193144
- DOI: 10.1084/jem.191.8.1381
Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine
Abstract
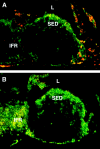
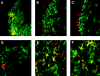
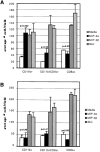
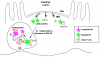
We describe the anatomical localization of three distinct dendritic cell (DC) subsets in the murine Peyer's patch (PP) and explore the role of chemokines in their recruitment. By two-color in situ immunofluorescence, CD11b(+) myeloid DCs were determined to be present in the subepithelial dome (SED) region, whereas CD8alpha(+) lymphoid DCs are present in the T cell-rich interfollicular region (IFR). DCs that lack expression of CD8alpha or CD11b (double negative) are present in both the SED and IFR. By in situ hybridization, macrophage inflammatory protein (MIP)-3alpha mRNA was dramatically expressed only by the follicle-associated epithelium overlying the SED, while its receptor, CCR6, was concentrated in the SED. In contrast, CCR7 was expressed predominantly in the IFR. Consistent with these findings, reverse transcriptase polymerase chain reaction analysis and in vitro chemotaxis assays using freshly isolated DCs revealed that CCR6 was functionally expressed only by DC subsets present in the SED, while all subsets expressed functional CCR7. Moreover, none of the splenic DC subsets migrated toward MIP-3alpha. These data support a distinct role for MIP-3alpha/CCR6 in recruitment of CD11b(+) DCs toward the mucosal surfaces and for MIP-3beta/CCR7 in attraction of CD8alpha(+) DCs to the T cell regions. Finally, we demonstrated that all DC subsets expressed an immature phenotype when freshly isolated and maintained expression of subset markers upon maturation in vitro. In contrast, CCR7 expression by myeloid PP DCs was enhanced with maturation in vitro. In addition, this subset disappeared from the SED and appeared in the IFR after microbial stimulation in vivo, suggesting that immature myeloid SED DCs capture antigens and migrate to IFR to initiate T cell responses after mucosal microbial infections.
Figures




References
-
- Spalding D.M., Griffin J.A. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986;44:507–515. - PubMed
-
- Ardavin C., Wu L., Li C.L., Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

