Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteers
- PMID: 10771448
- PMCID: PMC2015059
- DOI: 10.1046/j.1365-2125.2000.00147.x
Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteers
Abstract
Aims: To evaluate the pharmacokinetics and tolerability of single and multiple oral doses of ziprasidone in healthy male volunteers, and to determine the influence of ziprasidone on serum prolactin levels.
Methods: Single and multiple doses of ziprasidone were given orally (as two divided daily doses), at fixed dosages of 10 and 40 mg day(-1), and using titrated regimens of 40-80 and 40-120 mg day(-1), for 14 days. All dosages were taken immediately after food. The study adopted a randomized, double-blind, placebo-controlled design. Prolactin response, sedative properties, tolerability, and extrapyramidal symptoms were also investigated.
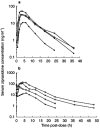
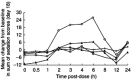
Results: Steady-state exposure to ziprasidone was attained after 1 day of dosing. Mean Cmax and AUC(0,12 h) increased with increasing dose, with apparent dose-proportionality between the 20 and 60 mg dose levels. Trough-to-peak ratios at steady state ranged from 2 to 5. Accumulation ratios for the fixed-dose regimens were 1.49 and 1.48 at the 5 and 20 mg dose levels, respectively. Ziprasidone was associated with transient prolactin elevation but levels of prolactin returned to baseline within the dosing interval at steady state. There was a marginal, transient increase in serum prolactin levels which was not dose-related at the 80 and 120 mg day(-1) doses, and which was noted to attenuate with chronic dosing. Ziprasidone was generally well tolerated. The most frequent side-effect was mild or moderate headache. A minority of patients suffered first-dose postural hypotension. Ziprasidone was also associated with a mild sedative effect that became less pronounced as treatment continued. There were no drug-related changes in electrocardiogram or clinical laboratory variables that were of clinical importance.
Conclusions: Ziprasidone is characterized by a predictable pharmacokinetic profile resulting in symptoms that reflect its pharmacological action.
Figures
References
-
- Seeger TF, Seymour PA, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. - PubMed
-
- Bersani G, Bressa GM, Meco G, et al. Combined serotonin 5HT2 and dopamine D2 antagonism in schizophrenia: clinical. Extrapyramidal and neuroendocrine response in a preliminary study with risperidone (R 64766) Hum Psychopharmacol. 1990;5:225–231.
-
- Metropolitan height and weight tables Stat Bull Metrop Life Found. 1983;64:3–9. - PubMed
-
- Janiszewski JS, Fouda HG, Cole RO. Development and validation of a high-sensitivity assay for an antipsychotic agent, CP-88,059, with solid-phase extraction and narrow-bone, high-performance liquid chromatography. J Chromatogr. 1995;668:133–139. - PubMed
-
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212(Suppl):11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

