Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions
- PMID: 10771452
- PMCID: PMC2015052
- DOI: 10.1046/j.1365-2125.2000.00151.x
Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions
Abstract
Aims: To identify the cytochrome P450 (CYP) isoform(s) responsible for the formation of the primary metabolite of ziprasidone (ziprasidone sulphoxide), to determine the kinetics of its formation and to predict possible drug interactions by investigating CYP isoform inhibition in an in vitro study.
Methods: In vitro metabolism of [14C]-ziprasidone was studied using human liver microsomes. The metabolites were identified using mass spectrometry. The kinetics of metabolite formation were determined using [14C]-ziprasidone (10-200 microM) over 5 min, and Km and Vmax were estimated from Lineweaver-Burk plots. IC50 values for the inhibition of specific probe substrates for CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, by ziprasidone, risperidone and 9-hydroxyrisperidone were also determined using human liver microsomes from three subjects. Mean Ki values were calculated.
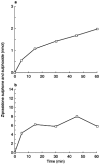
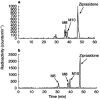
Results: Three CYP-mediated metabolites - ziprasidone sulphoxide, ziprasidone sulphone and oxindole acetic acid - were identified. The apparent Km and Vmax values for the formation of the major metabolite, ziprasidone sulphoxide (measured as the sum of sulphoxide and sulphone) were 235 microM and 1.14 nmol mg(-1) protein min(-1), respectively. Isoform-selective inhibitors and recombinant enzymes indicated that CYP3A4 is responsible for the formation of ziprasidone metabolites. Ziprasidone was not a substrate for the other isoforms studied. Similar in vitro inhibition of CYP2D6 (Ki 6.9-16 microM) and CYP3A4 (Ki 64-80 microM) was obtained with ziprasidone, risperidone and 9-hydroxyrisperidone. The in vivo free drug concentrations associated with clinically effective doses of ziprasidone are at least 1500-fold lower than the mean Ki for either CYP2D6 inhibition or CYP3A4 inhibition.
Conclusions: Ziprasidone is predominantly metabolized by CYP3A4 in human liver microsomes and is not expected to mediate drug interactions with coadministered CYP substrates, at clinically effective doses.
Figures


References
-
- Kamel A, Prakash C. Identification of in vitro metabolites of CP-88,059 in humans: characterization of metabolites by LC-MS/MS. Proceedings of the 43rd ASMS Conference on Mass Spectrometry and Allied Topics 887.
-
- Nelson DR, Kamataki T, Waxman DJ, et al. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names and nomenclature. DNA Cell Biol. 1993;12:1–51. - PubMed
-
- Gonzalez FJ. The molecular biology of cytochrome P450s. Pharmacol Rev. 1989;40:243–288. - PubMed
-
- Peck CC, Temple R, Collins JM. Understanding consequences of concurrent therapies. JAMA. 1993;269:1550–1552. - PubMed
-
- Tucker GT. The rational selection of drug interaction studies: implications of recent advances in drug metabolism. Int J Clin Pharmacol Ther Toxicol. 1992;30:550–553. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

