Ziprasidone and the pharmacokinetics of a combined oral contraceptive
- PMID: 10771454
- PMCID: PMC2015051
- DOI: 10.1046/j.1365-2125.2000.00153.x
Ziprasidone and the pharmacokinetics of a combined oral contraceptive
Abstract
Aims: To determine whether multiple doses of ziprasidone alter the steady-state pharmacokinetics of the component steroids, ethinyloestradiol and levonorgestrel, of an oral contraceptive; to evaluate the tolerability of a co-administered combined oral contraceptive and ziprasidone; and to compare plasma concentrations of prolactin in subjects taking a combined oral contraceptive with placebo or ziprasidone.
Methods: Nineteen women taking a combined oral contraceptive (ethinyloestradiol 30 microg day(-1) and levonorgestrel 150 microg day(-1)) were enrolled into a double-blind, placebo-controlled, two-way crossover study. They received ziprasidone 40 mg day- 1 in two divided daily doses or placebo for 8 days (days 8-15) in one of two 21 day treatment periods separated by a 7 day washout period. Venous blood samples were collected immediately before and up to 24 h after the morning dose of oral contraceptive and ziprasidone or placebo on day 15 of each 21 day treatment period. These were assayed for ethinyloestradiol and levonorgestrel and the resulting data used to derive pharmacokinetic data for these steroids. Additional samples were collected immediately before and 4 h after the morning dose of oral contraceptive and ziprasidone or placebo on day 15 of each 21 day treatment period for prolactin assay. All observed and volunteered adverse events were recorded throughout the study.
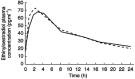
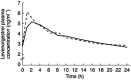
Results: The mean AUC(0,24 h), Cmax and tmax for ethinyloestradiol and the mean AUC(0, 24 h) and Cmax for levonorgestrel during ziprasidone co-administration were not statistically significantly different from corresponding values occurring during placebo co-administration. The tmax for levonorgestrel was approximately 0.5 h longer. Concomitant therapy with a combined oral contraceptive and ziprasidone did not result in adverse events not previously seen with either preparation alone.
Conclusions: The findings of this study suggest that, based on pharmacokinetic and tolerability data, ziprasidone may be co-administered with ethinyloestradiol and levonorgestrel without loss of contraceptive efficacy or increased risk of adverse events.
Figures
References
-
- Kulkarni J. Women and schizophrenia: a review. Austr NZ J Psychiatry. 1997;31:46–56. - PubMed
-
- Häfner H, van der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139–151. - PubMed
-
- Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr. 1992;20(Suppl):1–97. - PubMed
-
- Kendler KS, Walsh D. Gender and schizophrenia: results of an epidemiologically based family study. Br J Psychiatry. 1995;167:184–192. - PubMed
-
- Seeman MV, Lang M. The role of oestrogens in schizophrenia: gender differences. Schizophr Bull. 1990;16:185–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources