The pharmacokinetics of ziprasidone in healthy volunteers treated with cimetidine or antacid
- PMID: 10771455
- PMCID: PMC2015049
- DOI: 10.1046/j.1365-2125.2000.00154.x
The pharmacokinetics of ziprasidone in healthy volunteers treated with cimetidine or antacid
Abstract
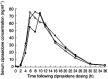
Aims: To evaluate the effects of cimetidine and Maalox(R) (aluminium hydroxide 1.35 g and magnesium hydroxide 1.2 g) on the pharmacokinetics of ziprasidone.
Methods: Eleven healthy young subjects aged 18-45 years were given single oral doses of ziprasidone 40 mg on three occasions at least 7 days apart. On one occasion ziprasidone was administered alone, on another occasion ziprasidone was co-administered with oral cimetidine 800 mg and on a third occasion ziprasidone was co-administered with oral Maalox(R).
Results: The administration of cimetidine increased the ziprasidone AUC(0,infinity) by 6% but there were no statistically significant differences in Cmax, tmax or lambda(z) between the ziprasidone+cimetidine group and the ziprasidone group. The administration of Maalox did not produce any statistically significant differences in AUC(0,infinity), Cmax, tmax or lambda(z) between the ziprasidone+Maalox group and the ziprasidone group.
Conclusions: The pharmacokinetics of ziprasidone are not affected by concurrent administration of cimetidine or Maalox. This suggests that other nonspecific inhibitors of cytochrome P450 and antacids are unlikely to alter the pharmacokinetics of ziprasidone.
Figures


References
-
- Lebel M, Proulx MJ, Allard S, et al. Influence of a high fat breakfast on the absorption and pharmacokinetics of CP-88,059, a new antipsychotic. Clin Invest Med. 1993;16:B18. Abstract 117.
-
- Knodell RG, Browne DG, Gwozdz GP, et al. Differential inhibition of individual human liver cytochrome P450 by cimetidine. Gastroenterology. 1991;101:1680–1691. - PubMed
-
- Metropolitan height and weight tables Stat Bull Metrop Life Found. 1983;64:3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

