The effects of ketoconazole on ziprasidone pharmacokinetics--a placebo-controlled crossover study in healthy volunteers
- PMID: 10771458
- PMCID: PMC2015056
- DOI: 10.1046/j.1365-2125.2000.00156.x
The effects of ketoconazole on ziprasidone pharmacokinetics--a placebo-controlled crossover study in healthy volunteers
Abstract
Aims: To assess the effects of multiple oral doses of ketoconazole on the single-dose pharmacokinetics of oral ziprasidone HCl.
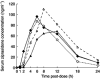
Methods: This was a 14-day, open-label, randomized, crossover study in 14 healthy subjects aged 18-31 years. Group 1 received oral ketoconazole 400 mg once daily for 6 days, followed by a 2 day wash-out period and 6 days of placebo administration. Group 2 received placebo followed by ketoconazole. Single oral doses of ziprasidone HCl 40 mg were administered on days 5 and 13 in both groups. Ziprasidone pharmacokinetic parameters were compared between placebo and ketoconazole administration periods.
Results: Co-administration of ziprasidone with ketoconazole was associated with a modest increase in ziprasidone exposure; mean ziprasidone AUC(0,infinity) increased by 33%, from 899 ng ml(-1) h with placebo to 1199 ng ml(-1) h with ketoconazole. Mean Cmax increased by 34%, from 89 ng ml(-1) to 119 ng ml(-1), respectively. The treatment effect on both of these parameters was statistically significant (P<0.02). Most adverse events were of mild intensity. There were no serious adverse events, laboratory abnormalities, abnormal ECGs, or clinically significant alterations in vital signs throughout the study.
Conclusions: The concurrent administration of ketoconazole and ziprasidone led to modest, statistically significant increases in ziprasidone exposure, although the changes seen were not considered clinically relevant. This suggests that other inhibitors of CYP3A4 are unlikely to significantly affect the pharmacokinetics of ziprasidone.
Figures
References
-
- Prakash C, Kamel A, Anderson W, Fouda H. Characterization of metabolites of CP-88 059 in rat using HPLC/RAM/ESI/MS/MS. Proceedings of the 42nd ASMS Conference on Mass Spectrometry and Allied Topics. Chicago, USA.
-
- Prakash C, Kamel A, Cui D, Whalen RD, Miceli JJ, Tweedie DJ. Ziprasidone metabolism and cytochrome P450 isoforms. Biol Psychiatry. 1997;42:40S.
-
- Prakash C, Kamel A, Cui D, Whalen RD, Miceli JJ, Tweedie D. Identification of the major human liver cytochrome P450 isoform (s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol. 2000;49(Suppl. 1):35S–42S. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources