Repair of tRNAs in metazoan mitochondria
- PMID: 10773071
- PMCID: PMC105380
- DOI: 10.1093/nar/28.10.2043
Repair of tRNAs in metazoan mitochondria
Abstract
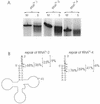
The integrity of 3'-ends of tRNAs is essential for aminoacylation and consequently for protein synthesis. The CCA-termini are generated and, if truncated by exonucleolytic activity, restored by tRNA nucleotidyltransferase. However, further truncations at the 3'-end can occur by exonuclease activity or during processing of overlapping tRNA primary transcripts in metazoan mitochondria. In the latter case, the upstream tRNA is released in a 3'-truncated form (lacking up to six bases) and subsequently completed. In human mitochondria, tRNA(Tyr)(missing the discriminator nucleotide A(73)) is completed by a discriminator adding activity followed by CCA addition. Since in vivo a high percentage of further 3'-terminally degraded human tRNA(Tyr)transcripts could be observed, it was tested in an in vitro system whether this repair mechanism for tRNA 3'-ends acts also on these further degraded tRNA versions. Additionally, 3'-truncated versions of two non-overlapping mitochondrial tRNAs (tRNA(Thr)and tRNA(Phe)) were examined. The results show that these transcripts can be repaired during incubation. A similar base incorporating activity was observed in mouse mitochondria, indicating that a repair mechanism for the 3'-end of several tRNAs exists in mitochondria of humans and possibly other metazoans which goes beyond the CCA addition.
Figures
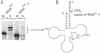
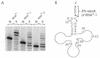
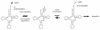
Similar articles
-
Mitochondrial poly(A) polymerase is involved in tRNA repair.Nucleic Acids Res. 2015 Nov 16;43(20):9937-49. doi: 10.1093/nar/gkv891. Epub 2015 Sep 9. Nucleic Acids Res. 2015. PMID: 26354863 Free PMC article.
-
Processing and editing of overlapping tRNAs in human mitochondria.J Biol Chem. 1998 Nov 27;273(48):31977-84. doi: 10.1074/jbc.273.48.31977. J Biol Chem. 1998. PMID: 9822669
-
Selective import of nuclear-encoded tRNAs into mitochondria of the protozoan Leishmania tarentolae.Mol Biochem Parasitol. 1993 Apr;58(2):233-45. doi: 10.1016/0166-6851(93)90045-y. Mol Biochem Parasitol. 1993. PMID: 8479448
-
Mitochondrial tRNA 3' end metabolism and human disease.Nucleic Acids Res. 2004 Oct 11;32(18):5430-41. doi: 10.1093/nar/gkh884. Print 2004. Nucleic Acids Res. 2004. PMID: 15477393 Free PMC article. Review.
-
tRNA biology in mitochondria.Int J Mol Sci. 2015 Feb 27;16(3):4518-59. doi: 10.3390/ijms16034518. Int J Mol Sci. 2015. PMID: 25734984 Free PMC article. Review.
Cited by
-
tRNA 5'-end repair activities of tRNAHis guanylyltransferase (Thg1)-like proteins from Bacteria and Archaea.Nucleic Acids Res. 2011 Mar;39(5):1833-42. doi: 10.1093/nar/gkq976. Epub 2010 Nov 3. Nucleic Acids Res. 2011. PMID: 21051361 Free PMC article.
-
Mitochondrial poly(A) polymerase is involved in tRNA repair.Nucleic Acids Res. 2015 Nov 16;43(20):9937-49. doi: 10.1093/nar/gkv891. Epub 2015 Sep 9. Nucleic Acids Res. 2015. PMID: 26354863 Free PMC article.
-
From end to end: tRNA editing at 5'- and 3'-terminal positions.Int J Mol Sci. 2014 Dec 22;15(12):23975-98. doi: 10.3390/ijms151223975. Int J Mol Sci. 2014. PMID: 25535083 Free PMC article. Review.
-
The complete mitochondrial genome of the entomopathogenic nematode Steinernema carpocapsae: insights into nematode mitochondrial DNA evolution and phylogeny.J Mol Evol. 2006 Feb;62(2):211-25. doi: 10.1007/s00239-005-0072-9. Epub 2006 Feb 10. J Mol Evol. 2006. PMID: 16474981
-
Large gene overlaps and tRNA processing in the compact mitochondrial genome of the crustacean Armadillidium vulgare.RNA Biol. 2015;12(10):1159-68. doi: 10.1080/15476286.2015.1090078. Epub 2015 Sep 11. RNA Biol. 2015. PMID: 26361137 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

