Domain specific interaction in the XRCC1-DNA polymerase beta complex
- PMID: 10773072
- PMCID: PMC105377
- DOI: 10.1093/nar/28.10.2049
Domain specific interaction in the XRCC1-DNA polymerase beta complex
Abstract
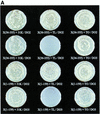
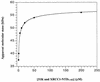
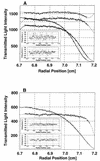
XRCC1 (X-ray cross-complementing group 1) is a DNA repair protein that forms complexes with DNA polymerase beta (beta-Pol), DNA ligase III and poly-ADP-ribose polymerase in the repair of DNA single strand breaks. The domains in XRCC1 have been determined, and characterization of the domain-domain interaction in the XRCC1-beta-Pol complex has provided information on the specificity and mechanism of binding. The domain structure of XRCC1, determined using limited proteolysis, was found to include an N-terminal domain (NTD), a central BRCT-I (breast cancer susceptibility protein-1) domain and a C-terminal BRCT-II domain. The BRCT-I-linker-BRCT-II C-terminal fragment and the linker-BRCT-II C-terminal fragment were relatively stable to proteolysis suggestive of a non-random conformation of the linker. A predicted inner domain was found not to be stable to proteolysis. Using cross-linking experiments, XRCC1 was found to bind intact beta-Pol and the beta-Pol 31 kDa domain. The XRCC1-NTD(1-183)(residues 1-183) was found to bind beta-Pol, the beta-Pol 31 kDa domain and the beta-Pol C-terminal palm-thumb (residues 140-335), and the interaction was further localized to XRCC1-NTD(1-157)(residues 1-157). The XRCC1-NTD(1-183)-beta-Pol 31 kDa domain complex was stable at high salt (1 M NaCl) indicative of a hydrophobic contribution. Using a yeast two-hybrid screen, polypeptides expressed from two XRCC1 constructs, which included residues 36-355 and residues 1-159, were found to interact with beta-Pol, the beta-Pol 31 kDa domain, and the beta-Pol C-terminal thumb-only domain polypeptides expressed from the respective beta-Pol constructs. Neither the XRCC1-NTD(1-159), nor the XRCC1(36-355)polypeptide was found to interact with a beta-Pol thumbless polypeptide. A third XRCC1 polypeptide (residues 75-212) showed no interaction with beta-Pol. In quantitative gel filtration and analytical ultracentrifugation experiments, the XRCC1-NTD(1-183)was found to bind beta-Pol and its 31 kDa domain in a 1:1 complex with high affinity (K(d) of 0.4-2.4 microM). The combined results indicate a thumb-domain specific 1:1 interaction between the XRCC1-NTD(1-159)and beta-Pol that is of an affinity comparable to other binding interactions involving beta-Pol.
Figures


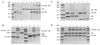

References
-
- Tebbs R.S., Flannery,M.L., Meneses,J.J., Hartmann,A., Tucker,J.D., Thompson,L.H., Cleaver,J.E. and Pedersen,R.A. (1999) Dev. Biol., 208, 513–529. - PubMed
-
- Sobol R.W., Horton,J.K., Kuhn,R., Gu,H., Singhal,R.K., Prasad,R., Rajewsky,K. and Wilson,S.H. (1996) Nature, 379, 183–186. - PubMed
-
- Thompson L.H. and West,M.G. (2000) Mutat. Res., 459, 1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

