Transcriptional control by the TGF-beta/Smad signaling system
- PMID: 10775259
- PMCID: PMC302010
- DOI: 10.1093/emboj/19.8.1745
Transcriptional control by the TGF-beta/Smad signaling system
Figures
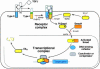
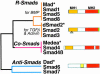


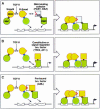
References
-
- Akiyoshi S., Inoue,H., Hanai,J., Kusanagi,K., Nemoto,N., Miyazono,K. and Kawabata,M. (1999) c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signaling through interaction with smads. J. Biol. Chem., 274, 35269–35277. - PubMed
-
- Ashcroft G.S. et al. (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nature Cell Biol., 1, 260–266. - PubMed
-
- Beckmann H., Su,L.K. and Kadesch,T. (1990) TFE3: a helix–loop–helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev., 4, 167–179. - PubMed
-
- Bertolino E., Reimund,B., Wildt-Perinic,D. and Clerc,R. (1995) A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J. Biol. Chem., 270, 31178–31188. - PubMed
-
- Brown C.B., Boyer,A.S., Runyan,R.B. and Barnett,J.V. (1999) Requirement of type III TGF-β receptor for endocardial cell transformation in the heart. Science, 283, 2080–2082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

