Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses
- PMID: 10775592
- PMCID: PMC111976
- DOI: 10.1128/jvi.74.10.4562-4569.2000
Multiple antiviral activities of cyanovirin-N: blocking of human immunodeficiency virus type 1 gp120 interaction with CD4 and coreceptor and inhibition of diverse enveloped viruses
Abstract
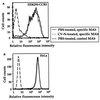
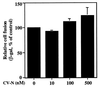
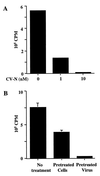
Cyanovirin-N (CV-N) is a cyanobacterial protein with potent neutralizing activity against human immunodeficiency virus (HIV). CV-N has been shown to bind HIV type 1 (HIV-1) gp120 with high affinity; moreover, it blocks the envelope glycoprotein-mediated membrane fusion reaction associated with HIV-1 entry. However, the inhibitory mechanism(s) remains unclear. In this study, we show that CV-N blocked binding of gp120 to cell-associated CD4. Consistent with this, pretreatment of gp120 with CV-N inhibited soluble CD4 (sCD4)-dependent binding of gp120 to cell-associated CCR5. To investigate possible effects of CV-N at post-CD4 binding steps, we used an assay that measures sCD4 activation of the HIV-1 envelope glycoprotein for fusion with CCR5-expressing cells. CV-N displayed equivalently potent inhibitory effects when added before or after sCD4 activation, suggesting that CV-N also has blocking action at the level of gp120 interaction with coreceptor. This effect was shown not to be due to CV-N-induced coreceptor down-modulation after the CD4 binding step. The multiple activities against the HIV-1 envelope glycoprotein prompted us to examine other enveloped viruses. CV-N potently blocked infection by feline immunodeficiency virus, which utilizes the chemokine receptor CXCR4 as an entry receptor but is CD4 independent. CV-N also inhibited fusion and/or infection by human herpesvirus 6 and measles virus but not by vaccinia virus. Thus, CV-N has broad-spectrum antiviral activity, both for multiple steps in the HIV entry mechanism and for diverse enveloped viruses. This broad specificity has implications for potential clinical utility of CV-N.
Figures




References
-
- Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. - PubMed
-
- Bewley C A, Gustafson K R, Boyd M R, Covell D G, Bax A, Clore G M, Gronenborn A M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat Struct Biol. 1998;5:571–578. - PubMed
-
- Boyd M R, Gustafson K R, McMohan J B, Shoemaker R H, O'Keefe B R, Mori T, Gulakowski R J, Wu L, Rivera M I, Laurencot C M, Currens M J, Cardellina I J H, Buckheit J R W, Nara P L, Pannell L K, Sowder I R C, Henderson L E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential application to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

