Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins
- PMID: 10775599
- PMCID: PMC111983
- DOI: 10.1128/jvi.74.10.4634-4644.2000
Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins
Abstract
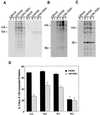
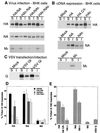
Influenza viruses encoding hemagglutinin (HA) and neuraminidase (NA) glycoproteins with deletions in one or both cytoplasmic tails (HAt- or NAt-) have a reduced association with detergent-insoluble glycolipids (DIGs). Mutations which eliminated various combinations of the three palmitoylation sites in HA exhibited reduced amounts of DIG-associated HA in virus-infected cells. The influenza virus matrix (M(1)) protein was also found to be associated with DIGs, but this association was decreased in cells infected with HAt- or NAt- virus. Regardless of the amount of DIG-associated protein, the HA and NA glycoproteins were targeted primarily to the apical surface of virus-infected, polarized cells. The uncoupling of DIG association and apical transport was augmented by the observation that the influenza A virus M(2) protein as well as the influenza C virus HA-esterase-fusion glycoprotein were not associated with DIGs but were apically targeted. The reduced DIG association of HAt- and NAt- is an intrinsic property of the glycoproteins, as similar reductions in DIG association were observed when the proteins were expressed from cDNA. Examination of purified virions indicated reduced amounts of DIG-associated lipids in the envelope of HAt- and NAt- viruses. The data indicate that deletion of both the HA and NA cytoplasmic tails results in reduced DIG association and changes in both virus polypeptide and lipid composition.
Figures





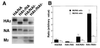


References
-
- Brown D A, Crise B, Rose J K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. - PubMed
-
- Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
-
- Burnet F M. Growth of influenza virus in the allantoic cavity of the chick embryo. Aust J Exp Biol Med Sci. 1941;19:291–295.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

