Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant
- PMID: 10775615
- PMCID: PMC111999
- DOI: 10.1128/jvi.74.10.4765-4775.2000
Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant
Abstract
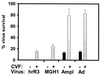
Intravascular routes of administration can provide a means to target gene- and virus-based therapies to multiple tumor foci located within an organ, such as the brain. However, we demonstrate here that rodent plasma inhibits cell transduction by replication-conditional (oncolytic) herpes simplex viruses (HSV), replication-defective HSV, and adenovirus vectors. In vitro depletion of complement with mild heat treatment or in vivo depletion by treatment of athymic rats with cobra venom factor (CVF) partially reverses this effect. Without CVF, inhibition of cell infection by HSV is observed at plasma dilution as high as 1:32, while plasma from CVF-treated animals displays anti-HSV activity at lower dilutions (1:8). When applied to the therapy of intracerebral brain tumors, in vivo complement depletion facilitates the initial infection (assayed at the 2-day time point) by an intra-arterial replication-conditional HSV of tumor cells, located within three separate and distinct human glioma masses. However, at the 4-day time point, no propagation of HSV from initially infected tumor cells could be observed. Previously, we have shown that the immunosuppressive agent, cyclophosphamide (CPA), facilitates the in vivo propagation of an oncolytic HSV, delivered intravascularly, within infected multiple intracerebral masses, by inhibition of both innate and elicited anti-HSV neutralizing antibody response (K. Ikeda et al., Nat. Med. 5:881-889, 1999). In this study, we thus show that the addition of CPA to the CVF treatment results in a significant increase in viral propagation within infected tumors, measured at the 4-day time period. The concerted action of CVF and CPA significantly increases the life span of athymic rodents harboring three separate and large glioma xenografts after treatment with intravascular, oncolytic HSV. Southern analysis of viral genomes analyzed by PCR reveals the presence of the oncolytic virus in the brains, livers, spleens, kidneys, and intestine of treated animals, although none of these tissues displays evidence of HSV-mediated gene expression. In light of clinical trials of oncolytic HSV for malignant brain tumors, these findings suggest that antitumor efficacy may be limited by the host innate and elicited humoral responses.
Figures








References
-
- Aghi M, Chou T C, Suling K, Breakefield X O, Chiocca E A. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865. - PubMed
-
- Anderson S C, Johnson D E, Harris M P, Engler H, Hancock W, Huang W M, Wills K N, Gregory R J, Sutjipto S, Wen S F, Lofgren S, Shepard H M, Maneval D C. p53 gene therapy in a rat model of hepatocellular carcinoma: intra-arterial delivery of a recombinant adenovirus. Clin Cancer Res. 1998;4:1649–1659. - PubMed
-
- Ash R J. Butyrate-induced reversal of herpes simplex virus restriction in neuroblastoma cells. Virology. 1986;155:584–592. - PubMed
-
- Ballow M, Cochrane C G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969;103:944–952. - PubMed
-
- Barnard R O, Geddes J F. The incidence of multifocal cerebral gliomas. A histologic study of large hemisphere sections. Cancer. 1987;60:1519–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

