H-1 parvovirus-associated replication bodies: a distinct virus-induced nuclear structure
- PMID: 10775619
- PMCID: PMC112003
- DOI: 10.1128/jvi.74.10.4807-4815.2000
H-1 parvovirus-associated replication bodies: a distinct virus-induced nuclear structure
Abstract
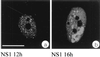
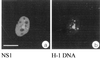
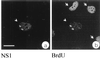
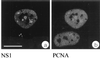
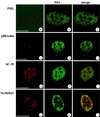
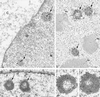
We have identified a nuclear structure that is induced after infection with the autonomous parvovirus H-1. Using fluorescence microscopy, we observed that the major nonstructural protein (NS1) of H-1 virus which is essential for viral DNA amplification colocalized with virus-specific DNA sequences and sites of ongoing viral DNA replication in distinct nuclear bodies which we designated H-1 parvovirus-associated replication bodies (H-1 PAR-bodies). In addition, two cellular proteins were shown to accumulate in H1 PAR-bodies: (i) the proliferating cell nuclear antigen (PCNA) which is essential for chromosomal and parvoviral replication and (ii) the NS1-interacting small glutamine-rich TPR-containing protein (SGT), suggesting a role for the latter in parvoviral replication and/or gene expression. Since many DNA viruses target preexisting nuclear structures, known as PML-bodies, for viral replication and gene expression, we have determined the localization of H-1 PAR- and PML-bodies by double-fluorescence labeling and confocal microscopy and found them to be spatially unrelated. Furthermore, H-1 PAR-bodies did not colocalize with other prominent nuclear structures such as nucleoli, coiled bodies, and speckled domains. Electron microscopy analysis revealed that NS1, as detected by indirect immunogold labeling, was localized in ring-shaped electron-dense nuclear structures corresponding in size and frequency to H-1 PAR-bodies. These structures were also clearly visible without immunogold labeling and could be detected only in infected cells. Our results suggest that H-1 virus does not target known nuclear bodies for DNA replication but rather induces the formation of a novel structure in the nucleus of infected cells.
Figures

Similar articles
-
Minute virus of mice NS1 interacts with the SMN protein, and they colocalize in novel nuclear bodies induced by parvovirus infection.J Virol. 2002 Apr;76(8):3892-904. doi: 10.1128/jvi.76.8.3892-3904.2002. J Virol. 2002. PMID: 11907229 Free PMC article.
-
Identification of a novel cellular TPR-containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1.J Virol. 1998 May;72(5):4149-56. doi: 10.1128/JVI.72.5.4149-4156.1998. J Virol. 1998. PMID: 9557704 Free PMC article.
-
Dynamics and interactions of parvoviral NS1 protein in the nucleus.Cell Microbiol. 2007 Aug;9(8):1946-59. doi: 10.1111/j.1462-5822.2007.00926.x. Epub 2007 Apr 5. Cell Microbiol. 2007. PMID: 17419720
-
[Advances in Parvovirus Non-structural Protein NS1 Induced Apoptosis].Bing Du Xue Bao. 2015 Nov;31(6):679-84. Bing Du Xue Bao. 2015. PMID: 26951015 Review. Chinese.
-
Concepts to Reveal Parvovirus-Nucleus Interactions.Viruses. 2021 Jul 5;13(7):1306. doi: 10.3390/v13071306. Viruses. 2021. PMID: 34372512 Free PMC article. Review.
Cited by
-
Parvovirus diversity and DNA damage responses.Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a012989. doi: 10.1101/cshperspect.a012989. Cold Spring Harb Perspect Biol. 2013. PMID: 23293137 Free PMC article. Review.
-
Expression and prognostic role of SGTA in human breast carcinoma correlates with tumor cell proliferation.J Mol Histol. 2014 Dec;45(6):665-77. doi: 10.1007/s10735-014-9586-z. Epub 2014 Jul 16. J Mol Histol. 2014. PMID: 25027991
-
Recruitment of DNA replication and damage response proteins to viral replication centers during infection with NS2 mutants of Minute Virus of Mice (MVM).Virology. 2011 Feb 20;410(2):375-84. doi: 10.1016/j.virol.2010.12.009. Epub 2010 Dec 30. Virology. 2011. PMID: 21193212 Free PMC article.
-
Replication Compartments of DNA Viruses in the Nucleus: Location, Location, Location.Viruses. 2020 Jan 29;12(2):151. doi: 10.3390/v12020151. Viruses. 2020. PMID: 32013091 Free PMC article. Review.
-
Efficient parvovirus replication requires CRL4Cdt2-targeted depletion of p21 to prevent its inhibitory interaction with PCNA.PLoS Pathog. 2014 Apr 3;10(4):e1004055. doi: 10.1371/journal.ppat.1004055. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24699724 Free PMC article.
References
-
- al-Lami F, Ledinko N, Toolan H W. Electron microscope study of human NB and SMH cells infected with the parvovirus H-1: involvement of the nucleolus. J Gen Virol. 1969;5:485–492.
-
- Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

