Liver-specific alpha 2 interferon gene expression results in protection from induced hepatitis
- PMID: 10775620
- PMCID: PMC112004
- DOI: 10.1128/jvi.74.10.4816-4823.2000
Liver-specific alpha 2 interferon gene expression results in protection from induced hepatitis
Abstract
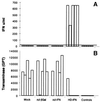
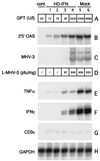
The current therapy for hepatitis B and C is based on systemic administration of recombinant human alpha interferon (r-hIFN-alpha). However, systemic delivery of r-hIFN-alpha is associated with severe side effects, but more importantly, it is effective in only a small percentage of patients. In an effort to maximize IFN-alpha antiviral efficacy, we have explored the therapeutic potential of murine IFN-alpha2 (mIFNalpha2) selectively expressed in the liver. To this end, we have developed a helper-dependent adenovirus vector (HD) containing the mIFN-alpha2 gene under the control of the liver-specific transthyretin promoter (HD-IFN). Comparison with a first-generation adenovirus carrying the same mIFN-alpha2 expression cassette indicates that at certain HD-IFN doses, induction of antiviral genes can be achieved in the absence of detectable circulating mIFN-alpha2. Challenge of injected mice with mouse hepatitis virus type 3 showed that HD-IFN provides high liver protection. Moreover, liver protection was also observed in acute nonviral liver inflammation hepatitis induced by concanavalin A at 1 month postinfection. These results hold promise for the development of a gene therapy treatment for chronic viral hepatitis based on liver-restricted expression of IFN-alpha2.
Figures




References
-
- Armstrong J A. Cytopathic effect inhibition assay for interferon: microculture plate assay. Methods Enzymol. 1981;78:381–387. - PubMed
-
- Ascione A, De Luca M, Canestrini C, Di Costanzo G G, Raimondo G, Longo G, Manns M P, Tillmann H L, Forte G B, Rocco P, Biceglia O, Faleo D, Vinelli F, Cela E M, Amitrano L, Addario L, Gigliotti T. Efficacy of high dose of recombinant alpha 2b interferon on long term response in chronic hepatitis C and cirrhosis: prospective randomized multicentre study. Ital J Gastroenterol Hepatol. 1998;30:517–523. - PubMed
-
- Bahr G M, Pouillart P R, Chedid L A. Enhancement in vivo of the antiinflammatory and antitumor activities of type I interferon by association with the synthetic immunomodulator murabutide. J Interferon Cytokine Res. 1996;16:297–306. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

