Evidence that G(z)-proteins couple to hypothalamic 5-HT(1A) receptors in vivo
- PMID: 10777773
- PMCID: PMC6773124
- DOI: 10.1523/JNEUROSCI.20-09-03095.2000
Evidence that G(z)-proteins couple to hypothalamic 5-HT(1A) receptors in vivo
Abstract
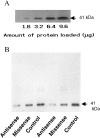
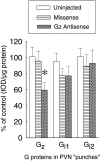
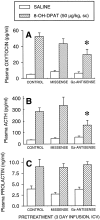
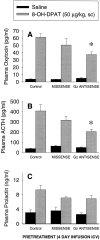
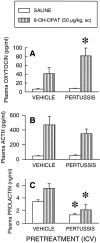
Using in situ hybridization and immunoblot analysis, the present studies identified G(z) mRNA and G(z)-protein in the hypothalamic paraventricular nucleus. The role of G(z)-proteins in hypothalamic 5-HT(1A) receptor signaling was examined in vivo. Activation of 5-HT(1A) receptors increases the secretion of oxytocin and ACTH, but not prolactin. Intracerebroventricular infusion (3-4 d) of G(z) antisense oligodeoxynucleotides, with different sequences and different phosphorothioate modification patterns, reduced the levels of G(z)-protein in the hypothalamic paraventricular nucleus, whereas missense oligodeoxynucleotides had no effect. Neither antisense nor missense oligodeoxynucleotide treatment altered basal plasma levels of ACTH, oxytocin, or prolactin, when compared with untreated controls. An antisense-induced decrease in hypothalamic G(z)-protein levels was paralleled by a significant decrease in the oxytocin and ACTH responses to the 5-HT(1A) agonist 8-hydroxy-dipropylamino-tetralin (8-OH-DPAT). In contrast, the prolactin response to 8-OH-DPAT (which cannot be blocked by 5-HT(1A) antagonists) was not inhibited by G(z) antisense oligodeoxynucleotides. G(z)-proteins are the only members of the G(i)/G(o)-protein family that are not inactivated by pertussis toxin. In a control experiment, pertussis toxin treatment (1 microgram/5 microliter, i.c.v.; 48 hr before the 8-OH-DPAT challenge) did not inhibit the ACTH response, potentiated the oxytocin response, and eliminated the prolactin response to 8-OH-DPAT. Thus, pertussis toxin-sensitive G(i)/G(o)-proteins do not mediate the 5-HT(1A) receptor-mediated increase in ACTH and oxytocin secretion. Combined, these studies provide the first in vivo evidence for a key role of G(z)-proteins in coupling hypothalamic 5-HT(1A) receptors to effector mechanisms.
Figures

Similar articles
-
Ovariectomy-induced increases in hypothalamic serotonin-1A receptor function in rats are prevented by estradiol.Neuroendocrinology. 2002 Dec;76(6):348-56. doi: 10.1159/000067582. Neuroendocrinology. 2002. PMID: 12566942
-
Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats.Neuroendocrinology. 2004;80(1):31-41. doi: 10.1159/000080795. Neuroendocrinology. 2004. PMID: 15385710
-
Sustained desensitization of hypothalamic 5-Hydroxytryptamine1A receptors after discontinuation of fluoxetine: inhibited neuroendocrine responses to 8-hydroxy-2-(Dipropylamino)Tetralin in the absence of changes in Gi/o/z proteins.J Pharmacol Exp Ther. 1999 Feb;288(2):561-7. J Pharmacol Exp Ther. 1999. PMID: 9918559
-
Selective breeding of 5-HT(1A) receptor-mediated responses: application to emotion and receptor action.Pharmacol Biochem Behav. 2000 Dec;67(4):701-8. doi: 10.1016/s0091-3057(00)00415-9. Pharmacol Biochem Behav. 2000. PMID: 11166060 Review.
-
Studies on the neuroendocrine role of serotonin.Dan Med Bull. 2007 Nov;54(4):266-88. Dan Med Bull. 2007. PMID: 18208678 Review.
Cited by
-
Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist.Br J Pharmacol. 2009 Jan;156(2):338-53. doi: 10.1111/j.1476-5381.2008.00001.x. Epub 2009 Jan 12. Br J Pharmacol. 2009. PMID: 19154445 Free PMC article.
-
Time-Restricted G-Protein Signaling Pathways via GPR176, Gz, and RGS16 Set the Pace of the Master Circadian Clock in the Suprachiasmatic Nucleus.Int J Mol Sci. 2020 Jul 17;21(14):5055. doi: 10.3390/ijms21145055. Int J Mol Sci. 2020. PMID: 32709014 Free PMC article. Review.
-
Tandospirone activates neuroendocrine and ERK (MAP kinase) signaling pathways specifically through 5-HT1A receptor mechanisms in vivo.Naunyn Schmiedebergs Arch Pharmacol. 2005 Jan;371(1):18-26. doi: 10.1007/s00210-004-1005-7. Epub 2005 Jan 18. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15655673
-
Gα Protein Signaling Bias at Serotonin 1A Receptor.Mol Pharmacol. 2023 Nov;104(5):230-238. doi: 10.1124/molpharm.123.000722. Epub 2023 Aug 11. Mol Pharmacol. 2023. PMID: 37567783 Free PMC article.
-
Gαz regulates BDNF-induction of axon growth in cortical neurons.Mol Cell Neurosci. 2014 Jan;58:53-61. doi: 10.1016/j.mcn.2013.12.004. Epub 2013 Dec 7. Mol Cell Neurosci. 2014. PMID: 24321455 Free PMC article.
References
-
- Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. - PubMed
-
- Aulakh CS, Wozniak KM, Haas M, Hill JL, Zohar J, Murphy DL. Food intake, neuroendocrine and temperature effects of 8-OHDPAT in the rat. Eur J Pharmacol. 1988;146:253–259. - PubMed
-
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. - PubMed
-
- Bagdy G. The role of biogenic amines in neuroendocrine regulation in conscious rats. In: Van de Kar LD, editor. Methods in neuroendocrinology. CRC; Boca Raton: 1998. pp. 145–161.
-
- Barr AJ, Brass LF, Manning DR. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor G protein coupling. J Biol Chem. 1997;272:2223–2229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
