Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons
- PMID: 10777782
- PMCID: PMC6773139
- DOI: 10.1523/JNEUROSCI.20-09-03182.2000
Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons
Abstract
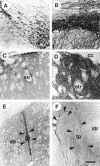
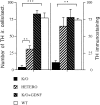
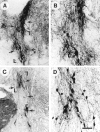
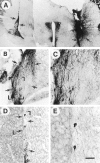
Glial cell line-derived neurotrophic factor (GDNF) is one of the most potent trophic factors that have been identified for midbrain dopamine (DA) neurons. Null mutations for trophic factor genes have been used frequently for studies of the role of these important proteins in brain development. One problem with these studies has been that often only prenatal development can be studied because many of the knockout strains, such as those with GDNF null mutations, will die shortly after birth. In this study, we looked at the continued fate of specific neuronal phenotypes from trophic factor knockout mice beyond the time that these animals die. By transplanting fetal neural tissues from GDNF -/-, GDNF +/-, and wild-type (WT) mice into the brain of adult wild-type mice, we demonstrate that the continued postnatal development of ventral midbrain dopamine neurons is severely disturbed as a result of the GDNF null mutation. Ventral midbrain grafts from -/- fetuses have markedly reduced DA neuron numbers and fiber outgrowth. Moreover, DA neurons in such transplants can be "rescued" by immersion in GDNF before grafting. These findings suggest that postnatal survival and/or phenotypic expression of ventral mesencephalic DA neurons is dependent on GDNF. In addition, we present here a strategy for studies of maturation and even aging of tissues from trophic factor and other knockout animals that do not survive past birth.
Figures

Similar articles
-
Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo.Exp Neurol. 1993 Dec;124(2):401-12. doi: 10.1006/exnr.1993.1214. Exp Neurol. 1993. PMID: 7904571
-
Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue.Exp Neurol. 1998 Oct;153(2):195-202. doi: 10.1006/exnr.1998.6884. Exp Neurol. 1998. PMID: 9784279
-
Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor.Cell Transplant. 2000 Jan-Feb;9(1):45-53. doi: 10.1177/096368970000900107. Cell Transplant. 2000. PMID: 10784066
-
Glial cell line-derived neurotrophic factor (GDNF): a drug candidate for the treatment of Parkinson's disease.J Neurol. 1998 Nov;245(11 Suppl 3):P35-42. doi: 10.1007/pl00007744. J Neurol. 1998. PMID: 9808338 Review.
-
Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
-
A dual-hit animal model for age-related parkinsonism.Prog Neurobiol. 2010 Feb 9;90(2):217-29. doi: 10.1016/j.pneurobio.2009.10.013. Epub 2009 Oct 21. Prog Neurobiol. 2010. PMID: 19853012 Free PMC article. Review.
-
Altered serum levels of glial cell line-derived neurotrophic factor in male chronic schizophrenia patients with tardive dyskinesia.Int J Methods Psychiatr Res. 2018 Dec;27(4):e1727. doi: 10.1002/mpr.1727. Epub 2018 Jun 14. Int J Methods Psychiatr Res. 2018. PMID: 29901253 Free PMC article.
-
PDLLA/β-TCP/HA/CHS/NGF Sustained-release Conduits for Peripheral Nerve Regeneration.J Wuhan Univ Technol Mater Sci Ed. 2021;36(4):600-606. doi: 10.1007/s11595-021-2450-6. Epub 2021 Aug 29. J Wuhan Univ Technol Mater Sci Ed. 2021. PMID: 34483596 Free PMC article.
-
Biological activities of astaxanthin in the treatment of neurodegenerative diseases.Neurodegener Dis Manag. 2024;14(6):241-256. doi: 10.1080/17582024.2024.2433932. Epub 2024 Dec 8. Neurodegener Dis Manag. 2024. PMID: 39648516 Review.
-
GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence.Alcohol Clin Exp Res. 2009 Jun;33(6):1012-24. doi: 10.1111/j.1530-0277.2009.00922.x. Epub 2009 Mar 19. Alcohol Clin Exp Res. 2009. PMID: 19302086 Free PMC article.
References
-
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberg F. Brain dopamine and the syndromes of Parkinson and Huntington. J Neurol Sci. 1973;20:415–455. - PubMed
-
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm A-C. Glial cell-line derived neurotrophic factor supports survival of injured midbrain DA neurons. J Comp Neurol. 1995;355:479–489. - PubMed
-
- Choi-Lundberg DL, Bohn M. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Dev Brain Res. 1995;85:80–88. - PubMed
-
- Clarkson ED, Zawada WM, Freed CR. GDNF improves survival and reduces apoptosis in human embryonic DA neurons in vitro. Cell Tissue Res. 1997;289:207–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
