Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures
- PMID: 10777787
- PMCID: PMC6773110
- DOI: 10.1523/JNEUROSCI.20-09-03221.2000
Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures
Abstract
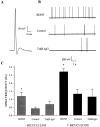
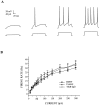
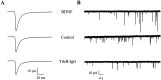
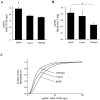
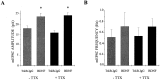
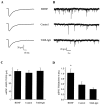
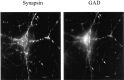
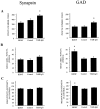
Brain-derived neurotrophic factor (BDNF) has been postulated to be a key signaling molecule in regulating synaptic strength and overall circuit activity. In this context, we have found that BDNF dramatically increases the frequency of spontaneously initiated action potentials in hippocampal neurons in dissociated culture. Using analysis of unitary synaptic transmission and immunocytochemical methods, we determined that chronic treatment with BDNF potentiates both excitatory and inhibitory transmission, but that it does so via different mechanisms. BDNF strengthens excitation primarily by augmenting the amplitude of AMPA receptor-mediated miniature EPSCs (mEPSCs) but enhances inhibition by increasing the frequency of mIPSC and increasing the size of GABAergic synaptic terminals. In contrast to observations in other systems, BDNF-mediated increases in AMPA-receptor mediated mEPSC amplitudes did not require activity, because blocking action potentials with tetrodotoxin for the entire duration of BDNF treatment had no effect on the magnitude of this enhancement. These forms of synaptic regulations appear to be a selective action of BDNF because intrinsic excitability, synapse number, and neuronal survival are not affected in these cultures. Thus, although BDNF induces a net increase in overall circuit activity, this results from potentiation of both excitatory and inhibitory synaptic drive through distinct and selective physiological mechanisms.
Figures

References
-
- Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol. 1996;76:4198–4201. - PubMed
-
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997a;272:32727–32730. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997b;276:2042–2045. - PubMed
-
- Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996;75:1250–1255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
