Intrinsic bias and lineage restriction in the phenotype determination of dopamine and neuropeptide Y amacrine cells
- PMID: 10777789
- PMCID: PMC6773102
- DOI: 10.1523/JNEUROSCI.20-09-03244.2000
Intrinsic bias and lineage restriction in the phenotype determination of dopamine and neuropeptide Y amacrine cells
Abstract
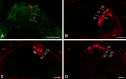
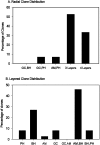
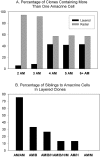
Blastomere lineages are differentially biased to produce different neurotransmitter subtypes of amacrine cells (Huang and Moody, 1995, 1997,). To elucidate when this bias is acquired, we examined amacrine lineages at different early developmental times. Our experiments demonstrate that the bias to express dopamine and neuropeptide Y amacrine fates involves several steps before the formation of the definitive optic cup. At cleavage stages, a retinal progenitor that contributes large numbers of cells is already biased to produce its normal repertoire of dopamine amacrine cells, as revealed by transplantation to a new location, whereas the amacrine fate of a progenitor that contributes fewer cells is modified by its new position. At neural plate stages, not all retinal progenitors are multipotent. Nearly one-half populate only the inner nuclear layer and are enriched in amacrine cells. During early optic vesicle stages, an appropriate mitotic tree is required for dopamine and neuropeptide Y, but not serotonin, amacrine cell clusters to form. Thus, the acquisition of amacrine fate bias involves intrinsic maternal factors at cleavage, fate restriction in the neural plate, and specified mitotic patterns in the optic vesicle. At each of these steps only a subset of the embryonic retinal progenitors contributing to amacrine subtypes is biased; the remaining progenitors maintain multipotency. Thus, from the earliest embryonic stages, progenitors of the retina are a dynamic mosaic. This is the first experimental demonstration of amacrine fate decisions that occur during early embryonic periods in advance of the events described in the later, committed retina.
Figures





Similar articles
-
Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus.Mol Vis. 2007 Jan 26;13:86-95. Mol Vis. 2007. PMID: 17277735 Free PMC article.
-
Asymmetrical blastomere origin and spatial domains of dopamine and neuropeptide Y amacrine subtypes in Xenopus tadpole retina.J Comp Neurol. 1995 Sep 25;360(3):442-53. doi: 10.1002/cne.903600306. J Comp Neurol. 1995. PMID: 8543650
-
The retinal fate of Xenopus cleavage stage progenitors is dependent upon blastomere position and competence: studies of normal and regulated clones.J Neurosci. 1993 Aug;13(8):3193-210. doi: 10.1523/JNEUROSCI.13-08-03193.1993. J Neurosci. 1993. PMID: 8340804 Free PMC article.
-
Emerging novel roles of neuropeptide Y in the retina: from neuromodulation to neuroprotection.Prog Neurobiol. 2014 Jan;112:70-9. doi: 10.1016/j.pneurobio.2013.10.002. Epub 2013 Oct 30. Prog Neurobiol. 2014. PMID: 24184719 Review.
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
Cited by
-
Reprogramming retinal pigment epithelium to differentiate toward retinal neurons with Sox2.Stem Cells. 2009 Jun;27(6):1376-87. doi: 10.1002/stem.48. Stem Cells. 2009. PMID: 19489100 Free PMC article.
-
bHLH genes and retinal cell fate specification.Mol Neurobiol. 2005 Oct;32(2):157-71. doi: 10.1385/MN:32:2:157. Mol Neurobiol. 2005. PMID: 16215280 Free PMC article. Review.
-
Origin and determination of inhibitory cell lineages in the vertebrate retina.J Neurosci. 2011 Feb 16;31(7):2549-62. doi: 10.1523/JNEUROSCI.4713-10.2011. J Neurosci. 2011. PMID: 21325522 Free PMC article.
-
Changes in Rx1 and Pax6 activity at eye field stages differentially alter the production of amacrine neurotransmitter subtypes in Xenopus.Mol Vis. 2007 Jan 26;13:86-95. Mol Vis. 2007. PMID: 17277735 Free PMC article.
-
Axon-bearing and axon-less horizontal cell subtypes are generated consecutively during chick retinal development from progenitors that are sensitive to follistatin.BMC Dev Biol. 2008 Apr 25;8:46. doi: 10.1186/1471-213X-8-46. BMC Dev Biol. 2008. PMID: 18439241 Free PMC article.
References
-
- Adelmann HB. Experimental studies on the development of the eye. IV. The effect of partial and complete excision of the prechordal substrata on the development of the eyes of Ambystoma punctatum. J Exp Zool. 1937;75:199–227.
-
- Adler R, Belecky-Adams T. Cell fate determination in the chick embryo retina. In: Moody SA, editor. Cell lineage and fate determination. Academic; New York: 1999. pp. 463–474.
-
- Alexiades MR, Cepko CL. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development. 1997;124:1119–1131. - PubMed
-
- Austin CP, Feldman DE, Ida JA, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. - PubMed
-
- Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann's Organizer: analysis of the movements of blastomere clones before and after gastrulation in Xenopus. Development. 1994;120:1179–1189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
