A spatiotemporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster Panulirus argus
- PMID: 10777792
- PMCID: PMC6773112
- DOI: 10.1523/JNEUROSCI.20-09-03282.2000
A spatiotemporal wave of turnover and functional maturation of olfactory receptor neurons in the spiny lobster Panulirus argus
Abstract
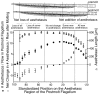
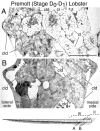
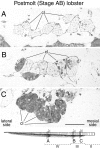
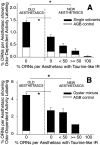
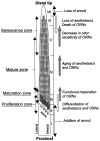
Olfactory receptor neurons (ORNs) of crustaceans are housed in aesthetasc sensilla that are located on the lateral flagellum of the antennule. We used young adult spiny lobsters to examine turnover of aesthetascs and functional maturation of their ORNs after molting. The proliferation zone for new aesthetascs is located in the proximal part of the aesthetasc-bearing region and progressively moves along a distoproximal axis. Older aesthetascs are lost in the distal part of the aesthetasc-bearing region. As a result, an aesthetasc may be shed three to six molts after it differentiates. Taurine-like immunoreactivity is elevated in ORNs of aesthetascs that have yet to emerge on the cuticular surface and thereafter decreases gradually and asynchronously. ORNs from the distalmost-developing aesthetascs lose taurine-like immunoreactivity immediately before sensillar emergence, whereas ORNs from the most proximal and lateral new aesthetascs retain taurine-like immunoreactivity throughout the intermolt stage after sensillar emergence. Furthermore, taurine-like immunoreactivity is inversely correlated with odor responsiveness. These results suggest that taurine-like immunoreactivity reveals immature ORNs and that their functional maturation is not synchronized with molting and may not be completed until many weeks after sensillar emergence. Our data suggest successive spatiotemporal waves of birth, differentiation and functional maturation, and death of ORNs.
Figures







Similar articles
-
Functional units of a compound nose: aesthetasc sensilla house similar populations of olfactory receptor neurons on the crustacean antennule.J Comp Neurol. 2000 Mar 13;418(3):270-80. J Comp Neurol. 2000. PMID: 10701826
-
Postembryonic proliferation in the spiny lobster antennular epithelium: rate of genesis of olfactory receptor neurons is dependent on molt stage.J Neurobiol. 2001 Apr;47(1):51-66. doi: 10.1002/neu.1015. J Neurobiol. 2001. PMID: 11257613
-
Dual antennular chemosensory pathways mediate odor-associative learning and odor discrimination in the Caribbean spiny lobster Panulirus argus.J Exp Biol. 2002 Mar;205(Pt 6):851-67. doi: 10.1242/jeb.205.6.851. J Exp Biol. 2002. PMID: 11914393
-
Learning from spiny lobsters about chemosensory coding of mixtures.Physiol Behav. 2000 Apr 1-15;69(1-2):203-9. doi: 10.1016/s0031-9384(00)00202-x. Physiol Behav. 2000. PMID: 10854930 Review.
-
Crustacean olfactory systems: A comparative review and a crustacean perspective on olfaction in insects.Prog Neurobiol. 2018 Feb;161:23-60. doi: 10.1016/j.pneurobio.2017.11.005. Epub 2017 Dec 2. Prog Neurobiol. 2018. PMID: 29197652 Review.
Cited by
-
Odorant responsiveness of squid olfactory receptor neurons.Anat Rec (Hoboken). 2008 Jul;291(7):763-74. doi: 10.1002/ar.20704. Anat Rec (Hoboken). 2008. PMID: 18484602 Free PMC article.
-
From Blood to Brain: Adult-Born Neurons in the Crayfish Brain Are the Progeny of Cells Generated by the Immune System.Front Neurosci. 2017 Dec 7;11:662. doi: 10.3389/fnins.2017.00662. eCollection 2017. Front Neurosci. 2017. PMID: 29270102 Free PMC article. Review.
-
Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection?Eur J Neurosci. 2011 Sep;34(6):870-83. doi: 10.1111/j.1460-9568.2011.07802.x. Eur J Neurosci. 2011. PMID: 21929622 Free PMC article. Review.
-
Adult neurogenesis in the central olfactory pathway in the absence of receptor neuron turnover in Libinia emarginata.Eur J Neurosci. 2005 Nov;22(10):2397-402. doi: 10.1111/j.1460-9568.2005.04449.x. Eur J Neurosci. 2005. PMID: 16307582 Free PMC article.
-
Adult neurogenesis and cell cycle regulation in the crustacean olfactory pathway: from glial precursors to differentiated neurons.J Mol Histol. 2007 Dec;38(6):527-42. doi: 10.1007/s10735-007-9112-7. Epub 2007 Jul 10. J Mol Histol. 2007. PMID: 17624620 Free PMC article.
References
-
- Altschuler D, Lo-Turco JJ, Rush J, Cepko C. Taurine promotes the differentiation of a vertebrate retinal cell type in vitro. Development. 1993;119:1317–1328. - PubMed
-
- Banerjee U, Zipursky SL. The role of cell-cell interaction in the development of the Drosophila visual system. Neuron. 1990;4:177–187. - PubMed
-
- Bode HR. Neuron determination in the ever-changing nervous system of Hydra. In: Shankland M, Macagno ER, editors. Determinants of neuronal identity. Academic; New York: 1992. pp. 323–357.
-
- Bodnaryk RP. Developmental changes in brain taurine levels during metamorphosis of the moth, Mamestra configurata. WLK Insect Biochem. 1981;11:9–16.
-
- Brézot P, Tauban D, Renou M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): sensillum types and numerical growth during the post-embryonic development. Int J Insect Morphol Embryol. 1997;25:427–441.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
