Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine
- PMID: 10777793
- PMCID: PMC6773125
- DOI: 10.1523/JNEUROSCI.20-09-03295.2000
Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine
Abstract
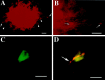
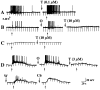
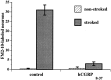
The enteric nervous system contains intrinsic primary afferent neurons that allow mucosal stimulation to initiate reflexes without CNS input. We tested the hypothesis that submucosal primary afferent neurons are activated by 5-hydroxytryptamine (5-HT) released from the stimulated mucosa. Fast and/or slow EPSPs were recorded in submucosal neurons after the delivery of exogenous 5-HT, WAY100325 (a 5-HT(1P) agonist), mechanical, or electrical stimuli to the mucosa of myenteric plexus-free preparations (+/- extrinsic denervation). These events were responses of second-order cells to transmitters released by excited primary afferent neurons. After all stimuli, fast and slow EPSPs were abolished by a 5-HT(1P) antagonist, N-acetyl-5-hydroxytryptophyl-5-hydroxytryptophan amide, and by 1.0 microM tropisetron, but not by 5-HT(4)-selective antagonists (SB204070 and GR113808A) or 5-HT(3)-selective antagonists (ondansetron and 0.3 microM tropisetron). Fast EPSPs in second-order neurons were blocked by hexamethonium, and most slow EPSPs were blocked by an antagonist of human calcitonin gene-related peptide (hCGRP(8-37)). hCGRP(8-37) also inhibited the spread of excitation in the submucosal plexus, assessed by measuring the uptake of FM2-10 and induction of c-fos. In summary, data are consistent with the hypothesis that 5-HT from enterochromaffin cells in response to mucosal stimuli initiates reflexes by stimulating 5-HT(1P) receptors on submucosal primary afferent neurons. Second-order neurons respond to these cholinergic/CGRP-containing cells with nicotinic fast EPSPs and/or CGRP-mediated slow EPSPs. Slow EPSPs are necessary for excitation to spread within the submucosal plexus. Because some second-order neurons contain also CGRP, primary afferent neurons may be multifunctional and also serve as interneurons.
Figures












Similar articles
-
Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity.J Neurosci. 1992 Jan;12(1):235-48. doi: 10.1523/JNEUROSCI.12-01-00235.1992. J Neurosci. 1992. PMID: 1729436 Free PMC article.
-
Organization of intrinsic cholinergic neurons projecting within submucosal plexus of guinea pig ileum.Am J Physiol. 1998 Sep;275(3):G490-7. doi: 10.1152/ajpgi.1998.275.3.G490. Am J Physiol. 1998. PMID: 9724260
-
Serotonin-induced increase in cAMP in ganglia isolated from the myenteric plexus of the guinea pig small intestine: mediation by a novel 5-HT receptor.Synapse. 1993 Apr;13(4):333-49. doi: 10.1002/syn.890130406. Synapse. 1993. PMID: 8386861
-
Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility.Aliment Pharmacol Ther. 2004 Nov;20 Suppl 7:3-14. doi: 10.1111/j.1365-2036.2004.02180.x. Aliment Pharmacol Ther. 2004. PMID: 15521849 Review.
-
Intrinsic primary afferent neurons of the intestine.Prog Neurobiol. 1998 Jan;54(1):1-18. doi: 10.1016/s0301-0082(97)00051-8. Prog Neurobiol. 1998. PMID: 9460790 Review.
Cited by
-
Morphine decreases enteric neuron excitability via inhibition of sodium channels.PLoS One. 2012;7(9):e45251. doi: 10.1371/journal.pone.0045251. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23028881 Free PMC article.
-
Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function.J Clin Invest. 2016 Jun 1;126(6):2221-35. doi: 10.1172/JCI84877. Epub 2016 Apr 25. J Clin Invest. 2016. PMID: 27111230 Free PMC article.
-
Excitation of rat colonic afferent fibres by 5-HT(3) receptors.J Physiol. 2002 Nov 1;544(3):861-9. doi: 10.1113/jphysiol.2002.025452. J Physiol. 2002. PMID: 12411529 Free PMC article.
-
Small bowel review: Normal physiology, part 2.Dig Dis Sci. 2003 Aug;48(8):1565-81. doi: 10.1023/a:1024724109128. Dig Dis Sci. 2003. PMID: 12924652 Review. No abstract available.
-
Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset.J Neurosci. 2004 Apr 28;24(17):4266-82. doi: 10.1523/JNEUROSCI.3688-03.2004. J Neurosci. 2004. PMID: 15115823 Free PMC article.
References
-
- Bertrand PP, Kunze WAA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;36:G422–G435. - PubMed
-
- Bertrand PP, Furness JB, Bornstein JC. 5-HT and ATP stimulate the mucosal terminals of some myenteric sensory neurons and cause increases in excitability in a population of sensory neurons. Falk Symposium No. 112. Neurogastroenterology: from the basics to the clinics. Freiburg, Germany; Falk Foundation: 1999.
-
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
-
- Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96:368–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
