Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons
- PMID: 10777798
- PMCID: PMC6773106
- DOI: 10.1523/JNEUROSCI.20-09-03354.2000
Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons
Abstract
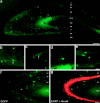
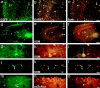
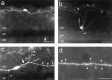
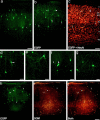
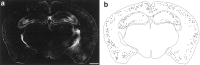
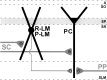
The chief inhibitory neurons of the mammalian brain, GABAergic neurons, are comprised of a myriad of diverse neuronal subtypes. To facilitate the study of these neurons, transgenic mice were generated that express enhanced green fluorescent protein (EGFP) in subpopulations of GABAergic neurons. In one of the resulting transgenic lines, called GIN (GFP-expressing Inhibitory Neurons), EGFP was found to be expressed in a subpopulation of somatostatin-containing GABAergic interneurons in the hippocampus and neocortex. In both live and fixed brain preparations from these mice, detailed microanatomical features of EGFP-expressing interneurons were readily observed. In stratum oriens of the hippocampus, EGFP-expressing interneurons were comprised almost exclusively of oriens/alveus interneurons with lacunosum-moleculare axon arborization (O-LM cells). In the neocortex, the somata of EGFP-expressing interneurons were largely restricted to layers II-IV and upper layer V. In hippocampal area CA1, two previously uncharacterized subtypes of interneurons were identified using the GIN mice: stratum pyramidale interneurons with lacunosum-moleculare axon arborization (P-LM cells) and stratum radiatum interneurons with lacunosum-moleculare axon arborization (R-LM cells). These newly identified interneuronal subtypes appeared to be closely related to O-LM cell, as they selectively innervate stratum lacunosum-moleculare. Whole-cell patch-clamp recordings revealed that these cells were fast-spiking and showed virtually no spike frequency accommodation. The microanatomical features of these cells suggest that they function primarily as "input-biasing" neurons, in that synaptic volleys in stratum radiatum would lead to their activation, which in turn would result in selective suppression of excitatory input from the entorhinal cortex onto CA1 pyramidal cells.
Figures




Similar articles
-
Signaling properties of stratum oriens interneurons in the hippocampus of transgenic mice expressing EGFP in a subset of somatostatin-containing cells.Hippocampus. 2007;17(7):538-53. doi: 10.1002/hipo.20291. Hippocampus. 2007. PMID: 17455332
-
Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro.J Physiol. 1998 Feb 1;506 ( Pt 3)(Pt 3):755-73. doi: 10.1111/j.1469-7793.1998.755bv.x. J Physiol. 1998. PMID: 9503336 Free PMC article.
-
Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border.Hippocampus. 2016 Sep;26(9):1107-14. doi: 10.1002/hipo.22611. Epub 2016 Jul 8. Hippocampus. 2016. PMID: 27328460 Free PMC article.
-
Neocortical inhibitory system.Folia Biol (Praha). 2009;55(6):201-17. Folia Biol (Praha). 2009. PMID: 20163769 Review.
-
Neurogliaform cells and other interneurons of stratum lacunosum-moleculare gate entorhinal-hippocampal dialogue.J Physiol. 2011 Apr 15;589(Pt 8):1875-83. doi: 10.1113/jphysiol.2010.201004. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135049 Free PMC article. Review.
Cited by
-
Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn.Ann N Y Acad Sci. 2013 Mar;1279:90-6. doi: 10.1111/nyas.12056. Ann N Y Acad Sci. 2013. PMID: 23531006 Free PMC article. Review.
-
Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex.Cereb Cortex. 2014 Aug;24(8):2237-48. doi: 10.1093/cercor/bht085. Epub 2013 Mar 31. Cereb Cortex. 2014. PMID: 23547136 Free PMC article.
-
Neuronal classification and marker gene identification via single-cell expression profiling of brainstem vestibular neurons subserving cerebellar learning.J Neurosci. 2012 Jun 6;32(23):7819-31. doi: 10.1523/JNEUROSCI.0543-12.2012. J Neurosci. 2012. PMID: 22674258 Free PMC article.
-
Vasculo-Neuronal Coupling: Retrograde Vascular Communication to Brain Neurons.J Neurosci. 2016 Dec 14;36(50):12624-12639. doi: 10.1523/JNEUROSCI.1300-16.2016. Epub 2016 Nov 7. J Neurosci. 2016. PMID: 27821575 Free PMC article.
-
Altered A-type potassium channel function in the nucleus tractus solitarii in acquired temporal lobe epilepsy.J Neurophysiol. 2019 Jan 1;121(1):177-187. doi: 10.1152/jn.00556.2018. Epub 2018 Dec 5. J Neurophysiol. 2019. PMID: 30517061 Free PMC article.
References
-
- Alonso A, Köhler C. Evidence for separate projections of hippocampal pyramidal and non-pyramidal neurons to different parts of the septum in the rat brain. Neurosci Lett. 1982;31:209–214. - PubMed
-
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
-
- Bergmann I, Nitsch R, Frotscher M. Area-specific morphological and neurochemical maturation of non-pyramidal neurons in the rat hippocampus as revealed by parvalbumin immunocytochemistry. Anat Embryol (Berl) 1991;184:403–404. - PubMed
-
- Bissette G. Neuropeptides and Alzheimer's disease pathology. Ann NY Acad Sci. 1997;814:17–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
