Specific and nonspecific effects of protein kinase C on the epithelial Na (+) channel
- PMID: 10779314
- PMCID: PMC2217227
- DOI: 10.1085/jgp.115.5.559
Specific and nonspecific effects of protein kinase C on the epithelial Na (+) channel
Abstract
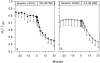
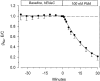
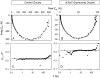
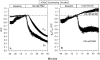
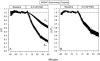
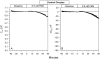
The Xenopus oocyte expression system was used to explore the mechanisms of inhibition of the cloned rat epithelial Na(+) channel (rENaC) by PKC (Awayda, M.S., I.I. Ismailov, B.K. Berdiev, C.M. Fuller, and D.J. Benos. 1996. J. Gen. Physiol. 108:49-65) and to determine whether human ENaC exhibits similar regulation. Effects of PKC activation on membrane and/or channel trafficking were determined using impedance analysis as an indirect measure of membrane area. hENaC-expressing oocytes exhibited an appreciable activation by hyperpolarizing voltages. This activation could be fit with a single exponential, described by a time constant (tau) and a magnitude (DeltaI (V)). A similar but smaller magnitude of activation was also observed in oocytes expressing rENaC. This activation likely corresponds to the previously described effect of hyperpolarizing voltage on gating of the native Na(+) channel (Palmer, L.G., and G. Frindt. 1996. J. Gen. Physiol. 107:35-45). Stimulation of PKC with 100 nM PMA decreased DeltaI(V) in hENaC-expressing oocytes to a plateau at 57.1 +/- 4.9% (n = 6) of baseline values at 20 min. Similar effects were observed in rENaC-expressing oocytes. PMA decreased the amiloride-sensitive hENaC slope conductance (g(Na)) to 21.7 +/- 7.2% (n = 6) of baseline values at 30 min. This decrease was similar to that previously reported for rENaC. This decrease of g (Na) was attributed to a decrease of membrane capacitance (C (m)), as well as the specific conductance (g(m)/C(m )). The effects on g(m)/C(m) reached a plateau within 15 min, at approximately 60% of baseline values. This decrease is likely due to the specific ability of PKC to inhibit ENaC. On the other hand, the decrease of C(m) was unrelated to ENaC and is likely an effect of PKC on membrane trafficking, as it was observed in ENaC-expressing as well as control oocytes. At lower PMA concentrations (0.5 nM), smaller changes of C(m) were observed in rENaC- and hENaC-expressing oocytes, and were preceded by larger changes of g(m ) and by changes of g(m)/C(m), indicating specific effects on ENaC. These findings indicate that PKC exhibits multiple and specific effects on ENaC, as well as nonspecific effects on membrane trafficking. Moreover, these findings provide the electrophysiological basis for assessing channel-specific effects of PKC in the Xenopus oocyte expression system.
Figures

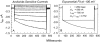



References
-
- Aballay A. , Stahl P.D. , Mayorga L.S. Phorbol ester promotes endocytosis by activating a factor involved in endosome fusion. J. Cell Sci. 1999 ;112:2549–2557 . - PubMed
-
- Aristimuno P.C. , Good D.W. PKC isoforms in rat medullary thick ascending limbselective activation of the δ-isoform by PGE2 . Am. J. Physiol. Renal Physiol. 1997 ;272:F624–F631 . - PubMed

