Repair of intermediate structures produced at DNA interstrand cross-links in Saccharomyces cerevisiae
- PMID: 10779332
- PMCID: PMC85635
- DOI: 10.1128/MCB.20.10.3425-3433.2000
Repair of intermediate structures produced at DNA interstrand cross-links in Saccharomyces cerevisiae
Abstract
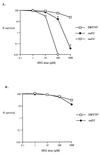
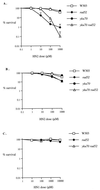
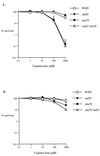
Bifunctional alkylating agents and other drugs which produce DNA interstrand cross-links (ICLs) are among the most effective antitumor agents in clinical use. In contrast to agents which produce bulky adducts on only one strand of the DNA, the cellular mechanisms which act to eliminate DNA ICLs are still poorly understood, although nucleotide excision repair is known to play a crucial role in an early repair step. Using haploid Saccharomyces cerevisiae strains disrupted for genes central to the recombination, nonhomologous end-joining (NHEJ), and mutagenesis pathways, all these activities were found to be involved in the repair of nitrogen mustard (mechlorethamine)- and cisplatin-induced DNA ICLs, but the particular pathway employed is cell cycle dependent. Examination of whole chromosomes from treated cells using contour-clamped homogenous electric field electrophoresis revealed the intermediate in the repair of ICLs in dividing cells, which are mostly in S phase, to be double-strand breaks (DSBs). The origin of these breaks is not clear since they were still efficiently induced in nucleotide excision and base excision repair-deficient, mismatch repair-defective, rad27 and mre11 disruptant strains. In replicating cells, RAD52-dependent recombination and NHEJ both act to repair the DSBs. In contrast, few DSBs were observed in quiescent cells, and recombination therefore seems dispensable for repair. The activity of the Rev3 protein (DNA polymerase zeta) is apparently more important for the processing of intermediates in stationary-phase cells, since rev3 disruptants were more sensitive in this phase than in the exponential growth phase.
Figures



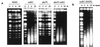
Similar articles
-
Repair of endonuclease-induced double-strand breaks in Saccharomyces cerevisiae: essential role for genes associated with nonhomologous end-joining.Genetics. 1999 Aug;152(4):1513-29. doi: 10.1093/genetics/152.4.1513. Genetics. 1999. PMID: 10430580 Free PMC article.
-
S. cerevisiae has three pathways for DNA interstrand crosslink repair.Mutat Res. 2001 Dec 19;487(3-4):73-83. doi: 10.1016/s0921-8777(01)00106-9. Mutat Res. 2001. PMID: 11738934
-
DNA interstrand cross-link repair in the Saccharomyces cerevisiae cell cycle: overlapping roles for PSO2 (SNM1) with MutS factors and EXO1 during S phase.Mol Cell Biol. 2005 Mar;25(6):2297-309. doi: 10.1128/MCB.25.6.2297-2309.2005. Mol Cell Biol. 2005. PMID: 15743825 Free PMC article.
-
Tying up loose ends: nonhomologous end-joining in Saccharomyces cerevisiae.Mutat Res. 2000 Jun 30;451(1-2):71-89. doi: 10.1016/s0027-5107(00)00041-5. Mutat Res. 2000. PMID: 10915866 Review.
-
Yeast base excision repair: interconnections and networks.Prog Nucleic Acid Res Mol Biol. 2001;68:29-39. doi: 10.1016/s0079-6603(01)68087-5. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554305 Review.
Cited by
-
Isolation of SOS constitutive mutants of Escherichia coli.J Bacteriol. 2004 Nov;186(21):7149-60. doi: 10.1128/JB.186.21.7149-7160.2004. J Bacteriol. 2004. PMID: 15489426 Free PMC article.
-
Cellular Repair of DNA-DNA Cross-Links Induced by 1,2,3,4-Diepoxybutane.Int J Mol Sci. 2017 May 18;18(5):1086. doi: 10.3390/ijms18051086. Int J Mol Sci. 2017. PMID: 28524082 Free PMC article.
-
Disruption of murine Mus81 increases genomic instability and DNA damage sensitivity but does not promote tumorigenesis.Mol Cell Biol. 2005 Sep;25(17):7569-79. doi: 10.1128/MCB.25.17.7569-7579.2005. Mol Cell Biol. 2005. PMID: 16107704 Free PMC article.
-
Rad5-dependent DNA repair functions of the Saccharomyces cerevisiae FANCM protein homolog Mph1.J Biol Chem. 2012 Aug 3;287(32):26563-75. doi: 10.1074/jbc.M112.369918. Epub 2012 Jun 12. J Biol Chem. 2012. PMID: 22696213 Free PMC article.
-
Editor's Highlight: High-Throughput Functional Genomics Identifies Modulators of TCE Metabolite Genotoxicity and Candidate Susceptibility Genes.Toxicol Sci. 2017 Nov 1;160(1):111-120. doi: 10.1093/toxsci/kfx159. Toxicol Sci. 2017. PMID: 28973557 Free PMC article.
References
-
- Averbeck D, Moustacchi E. 8-Methoxypsoralen plus 365nm light effects and repair in yeast. Biochim Biophys Acta. 1975;395:393–404. - PubMed
-
- Bai Y, Symington L S. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
