Overlapping expression of early B-cell factor and basic helix-loop-helix proteins as a mechanism to dictate B-lineage-specific activity of the lambda5 promoter
- PMID: 10779354
- PMCID: PMC85657
- DOI: 10.1128/MCB.20.10.3640-3654.2000
Overlapping expression of early B-cell factor and basic helix-loop-helix proteins as a mechanism to dictate B-lineage-specific activity of the lambda5 promoter
Abstract
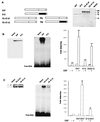
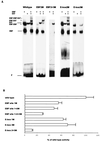
The basic helix-loop-helix (bHLH) transcription factors are a large group of proteins suggested to control key events in the development of B lymphocytes as well as of other cellular lineages. To examine how bHLH proteins activate a B-lineage-specific promoter, I investigated the ability of E47, E12, Heb, E2-2, and MyoD to activate the lambda5 surrogate light chain promoter. Comparison of the functional capacity of the E2A-encoded E47 and E12 proteins indicated that even though both were able to activate the lambda5 promoter and act in synergy with early B-cell factor (EBF), E47 displayed a higher functional activity than E12. An ability to act in synergy with EBF was also observed for Heb, E2-2, and MyoD, suggesting that these factors were functionally redundant in this regard. Mapping of functional domains in EBF and E47 revealed that the dimerization and DNA binding domains mediated the synergistic activity. Electrophoretic mobility shift assay analysis using the 5' part of the lambda5 promoter revealed formation of template-dependent heteromeric complexes between EBF and E47, suggesting that the synergistic mechanism involves cooperative binding to DNA. These findings propose a unique molecular function for E47 and provide overlapping expression with EBF as a molecular mechanism to direct B-cell-specific target gene activation by bHLH proteins.
Figures





Similar articles
-
EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes.Immunity. 1997 Jul;7(1):25-36. doi: 10.1016/s1074-7613(00)80507-5. Immunity. 1997. PMID: 9252117
-
Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12.J Exp Med. 1998 Aug 17;188(4):699-713. doi: 10.1084/jem.188.4.699. J Exp Med. 1998. PMID: 9705952 Free PMC article.
-
The human V-preB promoter is a target for coordinated activation by early B cell factor and E47.J Immunol. 2002 May 15;168(10):5130-8. doi: 10.4049/jimmunol.168.10.5130. J Immunol. 2002. PMID: 11994467
-
The lambda5-VpreB1 locus--a model system for studying gene regulation during early B cell development.Semin Immunol. 2005 Apr;17(2):121-7. doi: 10.1016/j.smim.2005.01.004. Semin Immunol. 2005. PMID: 15737573 Review.
-
E protein function in lymphocyte development.Annu Rev Immunol. 2002;20:301-22. doi: 10.1146/annurev.immunol.20.092501.162048. Epub 2001 Oct 4. Annu Rev Immunol. 2002. PMID: 11861605 Review.
Cited by
-
The EBF/Olf/Collier family of transcription factors: regulators of differentiation in cells originating from all three embryonal germ layers.Mol Cell Biol. 2002 Dec;22(24):8389-97. doi: 10.1128/MCB.22.24.8389-8397.2002. Mol Cell Biol. 2002. PMID: 12446759 Free PMC article. Review. No abstract available.
-
Early B cell factor: Regulator of B lineage specification and commitment.Semin Immunol. 2008 Aug;20(4):221-7. doi: 10.1016/j.smim.2008.07.004. Epub 2008 Aug 22. Semin Immunol. 2008. PMID: 18722139 Free PMC article. Review.
-
Induction of Epstein-Barr Virus Oncoprotein LMP1 by Transcription Factors AP-2 and Early B Cell Factor.J Virol. 2016 Mar 28;90(8):3873-3889. doi: 10.1128/JVI.03227-15. Print 2016 Apr. J Virol. 2016. PMID: 26819314 Free PMC article.
-
Coordinated transcriptional regulation of bone homeostasis by Ebf1 and Zfp521 in both mesenchymal and hematopoietic lineages.J Exp Med. 2013 May 6;210(5):969-85. doi: 10.1084/jem.20121187. Epub 2013 Apr 8. J Exp Med. 2013. PMID: 23569325 Free PMC article.
-
Prediction of Regulatory SNPs in Putative Minor Genes of the Neuro-Cardiovascular Variant in Fabry Reveals Insights into Autophagy/Apoptosis and Fibrosis.Biology (Basel). 2022 Aug 30;11(9):1287. doi: 10.3390/biology11091287. Biology (Basel). 2022. PMID: 36138766 Free PMC article.
References
-
- Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I, Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–1607. - PubMed
-
- Bain G, Maandag E C R, Izon D J, Amsen D, Kruisbeek A M, Weintraub B C, Kroop I, Schlissel M S, Feeney A J, van Roon M, van der Valk M, te Riel H P J, Berns A, Murre C. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. - PubMed
-
- Bain G, Maandag E C R, te Riele H P J, Feeney A J, Sheehy A, Schlissel M, Shinton S A, Hardy R R, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. - PubMed
-
- Bain G, Murre C. The role of E-proteins in B- and T-lymphocyte development. Semin Immunol. 1998;10:143–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
