Distinct phosphoisoforms of the Xenopus Mcm4 protein regulate the function of the Mcm complex
- PMID: 10779356
- PMCID: PMC85659
- DOI: 10.1128/MCB.20.10.3667-3676.2000
Distinct phosphoisoforms of the Xenopus Mcm4 protein regulate the function of the Mcm complex
Abstract
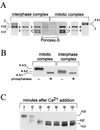
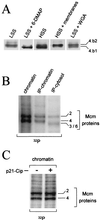
Initiation of DNA replication in eukaryotes requires the assembly of prereplication complexes (pre-Rcs) at the origins of replication. The assembly and function of the pre-Rcs appear to be controlled by phosphorylation events. In this study we report the detailed characterization of the cell cycle phosphorylation of one component of the Xenopus pre-Rcs, the Mcm protein complex. We show that individual Mcm subunits are differentially phosphorylated during the cell cycle. During mitosis, the Mcm4 subunit is hyperphosphorylated, while the other subunits are not actively phosphorylated. The mitotic phosphorylation of Mcm4 requires Cdc2-cyclin B and other unknown kinases. Following exit from mitosis, the Mcm4 subunit of the cytosolic interphase complex undergoes dephosphorylation, and the Mcm2, Mcm3, or Mcm6 subunits are then actively phosphorylated by kinase(s) other than cyclin-dependent kinases (Cdks) or Cdc7. The association of the Mcm complex with the pre-Rcs correlates with the formation of a transient interphase complex. This complex contains an intermediately phosphorylated Mcm4 subunit and is produced by partial dephosphorylation of the mitotic hyperphosphorylated Mcm4 protein. Complete dephosphorylation of the Mcm4 subunit inactivates the Mcm complex and prevents its binding to the chromatin. Once the Mcm complex is assembled on the chromatin the Mcm4 and the Mcm2 proteins are the only subunits phosphorylated during the activation of the pre-Rcs. These chromatin-associated phosphorylations require nuclear transport and are independent of Cdk2-cyclin E. These results suggest that the changes in Mcm4 phosphorylation regulate pre-Rc assembly and the function of the pre-Rcs on the chromatin.
Figures





References
-
- Aparicio O M, Weinstein D M, Bell S P. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell. 1997;91:59–69. - PubMed
-
- Bell S P, Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. - PubMed
-
- Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Brown G W, Kelly T J. Purification of Hsk1, a minichromosome maintenance protein kinase from fission yeast. J Biol Chem. 1998;273:22083–22090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
