Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline
- PMID: 10779551
- PMCID: PMC25821
- DOI: 10.1073/pnas.090020497
Xenopus kielin: A dorsalizing factor containing multiple chordin-type repeats secreted from the embryonic midline
Abstract
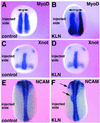
The midline tissues are important inductive centers of early vertebrate embryos. By signal peptide selection screening, we isolated a secreted factor, Kielin, which contains multiple cys-rich repeats similar to those in chordin (Chd). Expression of Kielin starts at midgastrula stages in the notochord and is detected in the floor plate of neurula embryos. Kielin is induced in mesoderm and in ectoderm by nodal-related genes. Chd is sufficient to activate Kielin expression in mesoderm whereas Shh or HNF-3beta in addition to Chd is required for induction in ectoderm. Kielin has a distinct biological activity from that of Chd. Injection of Kielin mRNA causes dorsalization of ventral marginal zone explants and expansion of MyoD expression in neurula embryos. Unlike Chd, Kielin does not efficiently induce neural differentiation of animal cap ectoderm, suggesting that the activity of Kielin is not simply caused by BMP4 blockade. Kielin is a signaling molecule that mediates inductive activities of the embryonic midline.
Figures





Similar articles
-
Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus.PLoS Biol. 2004 May;2(5):E92. doi: 10.1371/journal.pbio.0020092. Epub 2004 May 11. PLoS Biol. 2004. PMID: 15138495 Free PMC article.
-
Dorsalization of the neural tube by Xenopus tiarin, a novel patterning factor secreted by the flanking nonneural head ectoderm.Neuron. 2002 Feb 14;33(4):515-28. doi: 10.1016/s0896-6273(02)00590-1. Neuron. 2002. PMID: 11856527
-
Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development.Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12141-5. doi: 10.1073/pnas.92.26.12141. Proc Natl Acad Sci U S A. 1995. PMID: 8618860 Free PMC article.
-
Mesoderm induction by fibroblast growth factor in early Xenopus development.Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):75-84. doi: 10.1098/rstb.1990.0044. Philos Trans R Soc Lond B Biol Sci. 1990. PMID: 1969663 Review.
-
Conservation of dorsal-ventral patterning in arthropods and chordates.Curr Opin Genet Dev. 1996 Aug;6(4):424-31. doi: 10.1016/s0959-437x(96)80063-3. Curr Opin Genet Dev. 1996. PMID: 8791529 Review.
Cited by
-
Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing.Development. 2006 Jan;133(2):183-93. doi: 10.1242/dev.02214. Development. 2006. PMID: 16368928 Free PMC article. Review.
-
AmpA, a modular protein containing disintegrin and ornatin domains, has multiple effects on cell adhesion and cell fate specification.J Muscle Res Cell Motil. 2002;23(7-8):817-28. doi: 10.1023/a:1024440014857. J Muscle Res Cell Motil. 2002. PMID: 12952080 Review.
-
Agonists and Antagonists of TGF-β Family Ligands.Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a021923. doi: 10.1101/cshperspect.a021923. Cold Spring Harb Perspect Biol. 2016. PMID: 27413100 Free PMC article. Review.
-
The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila.Dev Cell. 2008 Jun;14(6):940-53. doi: 10.1016/j.devcel.2008.03.023. Dev Cell. 2008. PMID: 18539121 Free PMC article.
-
Highly-restricted, cell-specific expression of the simian CMV-IE promoter in transgenic zebrafish with age and after heat shock.Gene Expr Patterns. 2009 Jan;9(1):54-64. doi: 10.1016/j.gep.2008.07.002. Epub 2008 Aug 6. Gene Expr Patterns. 2009. PMID: 18723125 Free PMC article.
References
-
- Spemann H, Mangold H. Roux's Arch Entw Mech. 1924;100:599–638.
-
- De Robertis E M, Kim S, Leyns L, Piccolo S, Bachiller D, Agius E, Belo J A, Yamamoto A, Hainski-Brousseau A, Brizuela B, et al. Cold Spring Harbor Symp Quant Biol. 1997;XLII:169–175. - PubMed
-
- Smith W C, Harland R M. Cell. 1992;70:829–840. - PubMed
-
- Hemmati-Brivanlou A, Kelly O G, Melton D A. Cell. 1994;77:283–295. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

