Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses
- PMID: 10779552
- PMCID: PMC25877
- DOI: 10.1073/pnas.090034797
Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses
Abstract
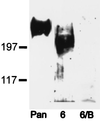
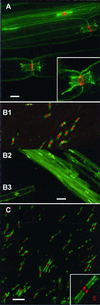
Voltage-gated sodium channels perform critical roles for electrical signaling in the nervous system by generating action potentials in axons and in dendrites. At least 10 genes encode sodium channels in mammals, but specific physiological roles that distinguish each of these isoforms are not known. One possibility is that each isoform is expressed in a restricted set of cell types or is targeted to a specific domain of a neuron or muscle cell. Using affinity-purified isoform-specific antibodies, we find that Na(v)1.6 is highly concentrated at nodes of Ranvier of both sensory and motor axons in the peripheral nervous system and at nodes in the central nervous system. The specificity of this antibody was also demonstrated with the Na(v)1.6-deficient mouse mutant strain med, whose nodes were negative for Na(v)1.6 immunostaining. Both the intensity of labeling and the failure of other isoform-specific antibodies to label nodes suggest that Na(v)1.6 is the predominant channel type in this structure. In the central nervous system, Na(v)1.6 is localized in unmyelinated axons in the retina and cerebellum and is strongly expressed in dendrites of cortical pyramidal cells and cerebellar Purkinje cells. Ultrastructural studies indicate that labeling in dendrites is both intracellular and on dendritic shaft membranes. Remarkably, Na(v)1.6 labeling was observed at both presynaptic and postsynaptic membranes in the cortex and cerebellum. Thus, a single sodium channel isoform is targeted to different neuronal domains and can influence both axonal conduction and synaptic responses.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

