Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice
- PMID: 10780957
- PMCID: PMC1571991
- DOI: 10.1038/sj.bjp.0703145
Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice
Abstract
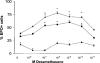
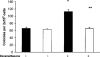
Since the production of eosinopoietic cytokines (GM-CSF, IL-3, IL-5) is inhibited by glucocorticoids, while responsiveness to these cytokines is enhanced in bone-marrow of allergic mice, we studied the ability of glucocorticoids to modulate murine bone-marrow eosinopoiesis. Progenitor (semi-solid) and/or precursor (liquid) cultures were established from bone-marrow of: (a) normal mice; (b) ovalbumin-sensitized and challenged mice or (c) dexamethasone (1-5 mg kg(-1)) injected mice. Cultures were established with GM-CSF (2 ng ml(-1)) or IL-5 (1 ng ml(-1)), respectively, alone or associated with dexamethasone, hydrocortisone or corticosterone. Total myeloid colony numbers, frequency and size of eosinophil colonies, and numbers of eosinophil-peroxidase-positive cells were determined at day 7. In BALB/c mice, dexamethasone (10(-7) M) increased GM-CSF-stimulated myeloid colony formation (P = 0.01), as well as the frequency (P=0.01) and size (P<0.01) of eosinophil colonies. Dexamethasone (10(-7) M) alone had no effect. Dexamethasone (10(-7)-10(-10) M) increased (P<0.002) eosinophil precursor responses to IL-5. Potentiation by dexamethasone was still detectable: (a) on low density, immature, nonadherent BALB/c bone-marrow cells, (b) on bone-marrow from other strains, and (c) on cells from allergic mice. Hydrocortisone and corticosterone had similar effects. Dexamethasone administered in vivo, 24 h before bone-marrow harvest, increased subsequent progenitor responses to GM-CSF (P = 0.001) and precursor responses to IL-5 (P<0.001). These effects were blocked by RU 486 (20 mg kg(-1), orally, 2 h before dexamethasone, or added in vitro at 10 microM, P<0.001). Glucocorticoids, acting in vivo or in vitro, through glucocorticoid receptors, enhance bone-marrow eosinopoiesis in naïve and allergic mice.
Figures
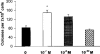

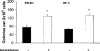

References
-
- AGARWAL S.K., MARSHALL G.D. Glucocorticoid-induced Type 1/Type 2 cytokine alterations in humans: a model for stress-related immune dysfunction. J. Interferon Cytokine Res. 1998;18:1059–1068. - PubMed
-
- BAGBY G.C.Hematopoiesis The Molecular Basis of Blood Diseases 1994W.B. Saunders, Philadelphia; 71–106.In: Stamatoyannopoulos, G., Nienhuis, A.W., Majerus, P.W., Varmus H. (eds.)(2nd. Ed.)
-
- BARNES P.J. Molecular mechanisms of steroid action in asthma. J. Allergy Clin. Immunol. 1996;97:159–168. - PubMed
-
- BARR D.D., VOLARIC Z., KLIM J.B. Stimulation of human eosinophilopoiesis by hydrocortisone in vitro. Acta Haematol. 1987;77:20–24. - PubMed
-
- BEGLEY C.G., BASSER R., MANSFIELD R., THOMSON B., PARKER W.R.L., LAYTON J., TO B., CEBON J., SHERIDAN W.P., FOX R.M., GREEN M.D. Enhanced levels and enhanced clonogenic capacity of blood progenitor cells following administration of stem cell factor plus granulocyte colony stimulating factor to humans. Blood. 1997;90:3378–3389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

